Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
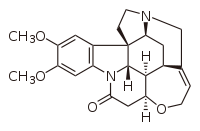
Brucine
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Dimethoxystrychnidin-10-one
| |
| Systematic IUPAC name
(4bR,4b1S,7aS,8aR,8a1R,12aS)-2,3-Dimethoxy-4b1,5,6,7a,8,8a,8a1,11,12a,13-decahydro-14H-12-oxa-7,14a-diaza-7,9-methanocyclohepta[cd]cyclopenta[g]fluoranthen-14-one | |
| Other names
2,3-Dimethoxystrychnine
10,11-Dimethoxystrychnine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.014 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1570 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H26N2O4 | |
| Molar mass | 394.471 g·mol−1 |
| Melting point | 178 °C (352 °F; 451 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H300, H330, H412 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P320, P321, P330, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Brucine is an alkaloid closely related to strychnine, most commonly found in the Strychnos nux-vomica tree. Brucine poisoning is rare, since it is usually ingested with strychnine, and strychnine is more toxic than brucine. In chemical synthesis, it can be used as a tool for stereospecific chemical syntheses.
Brucine's name derives from this of the genus Brucea, named after James Bruce who brought back Brucea antidysenterica from Ethiopia.
Previous Page Next Page


