
Back ميلامين Arabic ملامین AZB Меламін BE Меламин Bulgarian Melamina Catalan Melamin Czech Melamin Danish Melamin German Μελαμίνη Greek Melamino EO
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
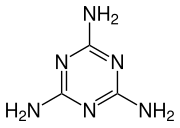
1,3,5-Triazine-2,4,6-triamine | |||
| Other names
2,4,6-Triamino-s-triazine
Cyanurotriamide Cyanurotriamine Cyanuramide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.288 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6N6 | |||
| Molar mass | 126.123 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.573 g/cm3[1] | ||
| Melting point | 343 °C (649 °F; 616 K) (decomposition)[1] | ||
| Boiling point | Sublimes | ||
| 3240 mg/ L (20 °C)[2] | |||
| Solubility | very slightly soluble in hot alcohol[clarification needed], benzene, glycerol, pyridine insoluble in ether, benzene, CCl4 | ||
| log P | −1.37 | ||
| Acidity (pKa) | 5.0 (conjugated acid)[3] | ||
| Basicity (pKb) | 9.0 [3] | ||
| −61.8·10−6 cm3/mol | |||
Refractive index (nD)
|
1.872[1] | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−1967 kJ/mol | ||
| Hazards | |||
| > 500 °C (932 °F; 773 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
3850 mg/kg (rat, oral) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

Melamine /ˈmɛləmiːn/ is an organic compound with the formula C3H6N6. This white solid is a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass, and its derivatives have fire-retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high pressure decorative laminates such as Formica, melamine dinnerware including cooking utensils, plates, plastic products,[4] laminate flooring, and dry erase boards. Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser.
Melamine-formaldehyde resin tableware was evaluated by the Taiwan Consumers' Foundation to have 20,000 parts per billion of free melamine that could migrate out of the plastic into acidic foods if held at 160 °F for two hours, such as if food was kept heated in contact with it in an oven.[4]
Melamine gained infamy because Chinese food producers added it to baby formula in order to increase the apparent protein content.[5][6] Ingestion of melamine may lead to reproductive damage, or bladder or kidney stones, and bladder cancer. It is also an irritant when inhaled or in contact with the skin or eyes. The United Nations' food standards body, the Codex Alimentarius Commission, has set the maximum amount of melamine allowed in powdered infant formula to 1 mg/kg and the amount of the chemical allowed in other foods and animal feed to 2.5 mg/kg. While not legally binding, the levels allow countries to ban importation of products with excessive levels of melamine.
- ^ a b c Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 3.516. ISBN 978-1439855119.
- ^ Melamine from PubChem
- ^ a b Jang, Y.H., Hwang, S., Chang, S.B., Ku, J. and Chung, D.S. (2009). "Acid Dissociation Constants of Melamine Derivatives from Density Functional Theory Calculations". The Journal of Physical Chemistry A. 113 (46): 13036–13040. Bibcode:2009JPCA..11313036J. doi:10.1021/jp9053583. PMID 19845385.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Nutrition, Center for Food Safety and Applied (December 19, 2022). "Melamine in Tableware Questions and Answers". FDA.
- ^ Hau, Anthony Kai-Ching; Kwan, Tze Hoi; Li, Philip Kam-tao (2009). "Melamine Toxicity and the Kidney". Journal of the American Society of Nephrology. 20 (2): 245–250. doi:10.1681/asn.2008101065. PMID 19193777.
- ^ Scholl, Peter F.; Bergana, Marti Mamula; Yakes, Betsy Jean; Xie, Zhuohong; Zbylut, Steven; Downey, Gerard; Mossoba, Magdi; Jablonski, Joseph; Magaletta, Robert; Holroyd, Stephen E.; Buehler, Martin (July 19, 2017). "Effects of the Adulteration Technique on the Near-Infrared Detection of Melamine in Milk Powder". Journal of Agricultural and Food Chemistry. 65 (28): 5799–5809. doi:10.1021/acs.jafc.7b02083. ISSN 0021-8561. PMID 28617599.

