
Back موميتازون فوروات Arabic مومتازون فوروات AZB Mometasonfuroat Danish Mometasonfuroat German Μομεταζόνη Greek Mometasona Spanish مومتازون فوروات FA Mométasone French Mometazon-furoát Hungarian Mometason ID
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nasonex, Asmanex, Elocon, others[1] |
| Other names | LAS-41002, 9α,21-Dichloro-11β,17α-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17α-(2-furoate) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical, inhalation (nasal spray) |
| Drug class | Corticosteroid; Glucocorticoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Nasal spray is virtually undetectable in plasma; but systemic availability is comparable to fluticasone[9] |
| Protein binding | 98% to 99% |
| Metabolism | Liver |
| Elimination half-life | 5.8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.600 |
| Chemical and physical data | |
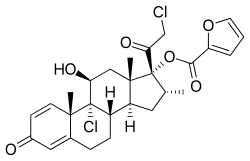
| Formula | C22H28Cl2O4 for mometasone C27H30O6Cl2 as furoate |
| 3D model (JSmol) |
|
| |
| |
| | |
Mometasone, also known as mometasone furoate, is a steroid (specifically, a glucocorticoid) medication used to treat certain skin conditions, hay fever, and asthma.[10][11][12] Specifically it is used to prevent rather than treat asthma attacks.[10] It can be applied to the skin, inhaled, or used in the nose.[10][11][12] Mometasone furoate, not mometasone, is used in medical products.[13]
Common side effects when used for asthma include headache, sore throat, and thrush.[10] It is therefore recommended to rinse the mouth after use.[10] Long-term use may increase the risk for glaucoma and cataracts.[10] Common side effects when used in the nose include upper respiratory tract infections and nose bleeds.[12] Common side effects when applied on the skin include acne, skin atrophy, and itchiness.[11] It works by decreasing inflammation.[10]
Mometasone furoate was patented in 1981 and came into medical use in 1987.[14] It is on the World Health Organization's List of Essential Medicines[15] and is available as a generic medication.[16][17] In 2022, it was the 311th most commonly prescribed medication in the United States, with more than 200,000 prescriptions.[18]
- ^ Cite error: The named reference
brands2016was invoked but never defined (see the help page). - ^ "Mometasone Use During Pregnancy". Drugs.com. 14 February 2020. Archived from the original on 26 October 2020. Retrieved 1 April 2020.
- ^ "Nasonex Allergy mometasone furoate 50 micrograms/ actuation (as monohydrate) aqueous nasal spray (215798)". Therapeutic Goods Administration (TGA). 15 July 2020. Retrieved 22 August 2024.
- ^ "Nasonex aqueous nasal spray mometasone furoate 50 microgram/actuation (as monohydrate) spray bottle (77112)". Therapeutic Goods Administration (TGA). 10 October 2023. Retrieved 22 August 2024.
- ^ "Noumed Mometasone Hayfever & Allergy Relief (Noumed Pharmaceuticals PTY LTD)". Therapeutic Goods Administration (TGA). 13 September 2024. Retrieved 15 September 2024.
- ^ "Nasonex- mometasone furoate spray, metered". DailyMed. 26 January 2011. Archived from the original on 5 November 2022. Retrieved 19 June 2022.
- ^ "Nasonex- mometasone furoate spray". DailyMed. 15 December 2022. Archived from the original on 17 April 2023. Retrieved 17 April 2023.
- ^ "List of nationally authorised medicinal products" (PDF). European Medicines Agency. Archived (PDF) from the original on 7 March 2023. Retrieved 6 March 2023.
- ^ Tayab ZR, Fardon TC, Lee DK, Haggart K, McFarlane LC, Lipworth BJ, et al. (November 2007). "Pharmacokinetic/pharmacodynamic evaluation of urinary cortisol suppression after inhalation of fluticasone propionate and mometasone furoate". British Journal of Clinical Pharmacology. 64 (5): 698–705. doi:10.1111/j.1365-2125.2007.02919.x. PMC 2203259. PMID 17509041.
- ^ a b c d e f g "Mometasone Furoate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 7 October 2016. Retrieved 11 March 2019.
- ^ a b c "Mometasone Furoate topical Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 5 August 2020. Retrieved 11 March 2019.
- ^ a b c "Mometasone Furoate eent Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 30 April 2016. Retrieved 11 March 2019.
- ^ "Mometasone". DrugBank. Archived from the original on 29 June 2019. Retrieved 30 April 2020.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 488. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 265. ISBN 9780857113382.
- ^ "Mometasone Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.