
Back موكسيفلوكساسين Arabic موکسی فلوکساسین AZB মক্সিফ্লক্সাসিন Bengali/Bangla Moxifloxacina Catalan Mocsifflocsacin CY Moxifloxacin German Μοξιφλοξασίνη Greek Moxifloxacino Spanish Moxifloxazino EU موکسی فلوکساسین FA
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avelox, Vigamox, Moxiflox, others |
| Other names | Moxifloxacine; BAY 12-8039 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, eye drops |
| Drug class | Antibiotic (fluoroquinolone) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 86%[2] |
| Protein binding | 47%[2] |
| Metabolism | Glucuronide and sulfate conjugation; CYP450 system not involved[3] |
| Elimination half-life | 12.1 hours[2] |
| Excretion | Urine, feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.129.459 |
| Chemical and physical data | |
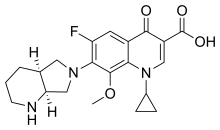
| Formula | C21H24FN3O4 |
| Molar mass | 401.438 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Moxifloxacin is an antibiotic, used to treat bacterial infections,[4] including pneumonia, conjunctivitis, endocarditis, tuberculosis, and sinusitis.[4][5] It can be given by mouth, by injection into a vein, and as an eye drop.[5]
Common side effects include diarrhea, dizziness, and headache.[4] Severe side effects may include spontaneous tendon ruptures, nerve damage, and worsening of myasthenia gravis.[4] Safety of use in pregnancy and breastfeeding is unclear.[6] Moxifloxacin is in the fluoroquinolone family of medications.[4] It usually kills bacteria by blocking their ability to duplicate DNA.[4]
Moxifloxacin was patented in 1988 and approved for use in the United States in 1999.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] In 2022, it was the 273rd most commonly prescribed medication in the United States, with more than 800,000 prescriptions.[10][11]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c Zhanel GG, Fontaine S, Adam H, Schurek K, Mayer M, Noreddin AM, et al. (2006). "A Review of New Fluoroquinolones : Focus on their Use in Respiratory Tract Infections". Treatments in Respiratory Medicine. 5 (6): 437–465. doi:10.2165/00151829-200605060-00009. PMID 17154673. S2CID 26955572.
- ^ World Health Organization (2008). Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. World Health Organization. pp. 189–. ISBN 978-92-4-154758-1.
- ^ a b c d e f "Moxifloxacin Hydrochloride". The American Society of Health-System Pharmacists. Retrieved 29 August 2017.
- ^ a b British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 408, 757. ISBN 9780857111562.
- ^ "Moxifloxacin Use During Pregnancy". Drugs.com. Retrieved 10 December 2017.
- ^ "Details for NDA:021085". DrugPatentWatch. Retrieved 17 July 2009.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 501. ISBN 9783527607495.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Moxifloxacin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.