Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
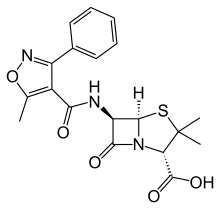
Oxacillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Bactocill |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685020 |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.577 |
| Chemical and physical data | |
| Formula | C19H19N3O5S |
| Molar mass | 401.44 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.49 g/cm3 |
| Boiling point | 686.8 °C (1,268.2 °F) |
| |
| |
| | |
Oxacillin (trade name Bactocill) is a narrow-spectrum second-generation beta-lactam antibiotic of the penicillin class developed by Beecham.[1]
It was patented in 1960 and approved for medical use in 1962.[2]
- ^ Greenwood D (2008). Antimicrobial drugs: chronicle of a twentieth century medical triumph. Oxford University Press US. pp. 124–. ISBN 978-0-19-953484-5. Retrieved 18 November 2010.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 490. ISBN 9783527607495.
Previous Page Next Page


