
Back أوكسي ميتازولين Arabic اوکسی متازولین AZB Ocsymetasolin CY Oxymetazolin German Oximetazolina Spanish اوکسی متازولین FA Oxymétazoline French אוקסימטזולין HE Oximetazolin Hungarian Oksimetazolin ID
 | |
| Clinical data | |
|---|---|
| Trade names | Afrin, others |
| AHFS/Drugs.com | Monograph |
| Dependence liability | Moderate |
| Routes of administration | Intranasal, eye drop, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5–6 hours |
| Excretion | Kidney: 30% Feces: 10% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.618 |
| Chemical and physical data | |
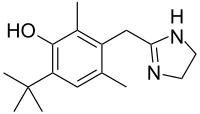
| Formula | C16H24N2O |
| Molar mass | 260.381 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 182 °C (360 °F) |
| |
| |
| | |
Oxymetazoline, sold under the brand name Afrin among others, is a topical decongestant and vasoconstrictor medication. It is available over-the-counter as a nasal spray to treat nasal congestion and nosebleeds, as eye drops to treat eye redness due to minor irritation, and (in the United States) as a prescription topical cream to treat persistent facial redness due to rosacea in adults. Its effects begin within minutes and last for up to six hours. Intranasal use for longer than three to five days may cause congestion to recur or worsen, resulting in physical dependence.
Oxymetazoline is a derivative of imidazole.[1] It was developed from xylometazoline at Merck by Wolfgang Fruhstorfer and Helmut Müller-Calgan in 1961.[2] A direct sympathomimetic, oxymetazoline binds to and activates α1 adrenergic receptors and α2 adrenergic receptors, most notably.[1] One study classified it in the following order: α(2A) > α(1A) ≥ α(2B) > α(1D) ≥ α(2C) >> α(1B), but this is not universally agreed upon.[3]
Another study classified it with selectivity ratios in alpha 2 adrenergic receptors of 200 for a2A vs a2B, 7.1 a2A vs a2C, and 28.2 a2B vs a2C.[4]
In 2022, it was the 305th most commonly prescribed medication in the United States, with more than 300,000 prescriptions.[5]
- ^ a b "Oxymetazoline". PubChem. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. CID 4636.
- ^ DE 1117588, Fruhstorfer W, Müller-Calgan H, "2-(2,6-dimethyl-3-hydroxy-4-tert-butyl-benzyl)-2-imidazoline,and acid addition salts thereof,and process for their manufacture", issued 23 November 1961, assigned to E Merck AG.
- ^ Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R, et al. (December 2010). "Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline". Fundamental & Clinical Pharmacology. 24 (6): 729–739. doi:10.1111/j.1472-8206.2009.00805.x. PMID 20030735. S2CID 25064699.
- ^ Proudman RG, Akinaga J, Baker JG (October 2022). "The signaling and selectivity of α-adrenoceptor agonists for the human α2A, α2B and α2C-adrenoceptors and comparison with human α1 and β-adrenoceptors". Pharmacology Research & Perspectives. 10 (5): e01003. doi:10.1002/prp2.1003. PMC 9471048. PMID 36101495.
- ^ "Oxymetazoline Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.