Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
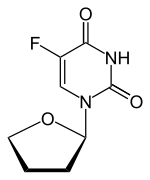
Tegafur
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 5-fluoro-1-(oxolan-2-yl)pyrimidine-2,4-dione |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 3.9-11 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.038.027 |
| Chemical and physical data | |
| Formula | C8H9FN2O3 |
| Molar mass | 200.169 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tegafur is a chemotherapeutic prodrug of 5-fluorouracil (5-FU) used in the treatment of cancers. It is a component of the combination drug tegafur/uracil. When metabolised, it becomes 5-FU.[1]
It was patented in 1967 and approved for medical use in 1972.[2]
- ^ El Sayed YM, Sadée W (September 1983). "Metabolic activation of R,S-1-(tetrahydro-2-furanyl)-5-fluorouracil (ftorafur) to 5-fluorouracil by soluble enzymes". Cancer Research. 43 (9): 4039–4044. PMID 6409396.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 511. ISBN 9783527607495.
Previous Page Next Page


