Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
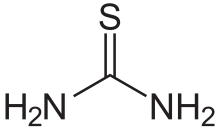
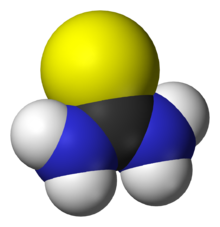
Thiourea
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Thiourea[1] | |
| Other names
Thiocarbamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| 605327 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.494 |
| 1604 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2811 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SC(NH2)2 | |
| Molar mass | 76.12 g·mol−1 |
| Appearance | white solid |
| Density | 1.405 g/mL |
| Melting point | 182 °C (360 °F; 455 K) |
| 142 g/L (25 °C) | |
| −4.24×10−5 cm3/mol | |
| Hazards | |
| GHS labelling: | |
  
| |
| Warning | |
| H302, H351, H361, H411 | |
| P201, P202, P264, P270, P273, P281, P301+P312, P308+P313, P330, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Urea Selenourea |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thiourea (/ˌθaɪ.oʊjʊəˈriː.ə, -ˈjʊəri-/)[2][3][4] is an organosulfur compound with the formula SC(NH2)2 and the structure H2N−C(=S)−NH2. It is structurally similar to urea (H2N−C(=O)−NH2), except that the oxygen atom is replaced by a sulfur atom (as implied by the thio- prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. Thioureas are a broad class of compounds with the general structure R2N−C(=S)−NR2.
- ^ Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. pp. 98, 864. doi:10.1039/9781849733069. ISBN 9780854041824. OCLC 1077224056.
- ^ "thiourea". Lexico UK English Dictionary. Oxford University Press. Archived from the original on 2020-03-22.
- ^ "thiourea". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 2016-01-21.
- ^ "thiourea". Dictionary.com Unabridged (Online). n.d.
Previous Page Next Page



