Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Atomic layer deposition
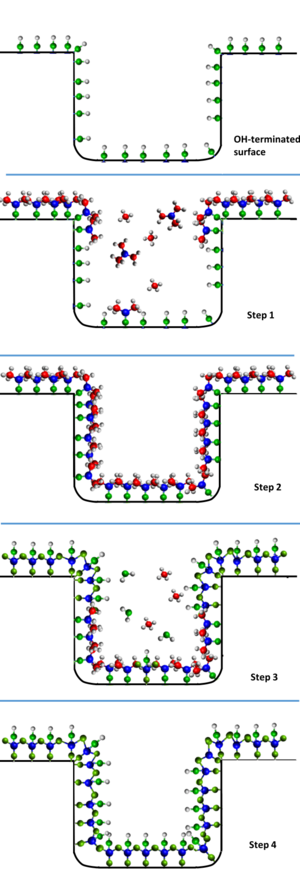
Atomic layer deposition (ALD) is a thin-film deposition technique based on the sequential use of a gas-phase chemical process; it is a subclass of chemical vapour deposition. The majority of ALD reactions use two chemicals called precursors (also called "reactants"). These precursors react with the surface of a material one at a time in a sequential, self-limiting, manner. A thin film is slowly deposited through repeated exposure to separate precursors. ALD is a key process in fabricating semiconductor devices, and part of the set of tools for synthesizing nanomaterials.
- ^ Cremers, Véronique; Puurunen, Riikka L.; Dendooven, Jolien (4 April 2019). "Conformality in atomic layer deposition: Current status overview of analysis and modelling". Applied Physics Reviews. 6 (2). AIP Publishing (published June 2019). doi:10.1063/1.5060967. eISSN 1931-9401. hdl:1854/LU-8614054.
Previous Page Next Page


