Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
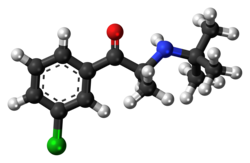
Bupropion
 | |
 1 : 1 mixture (racemate) | |
| Clinical data | |
|---|---|
| Pronunciation | /bjuːˈproʊpiɒn/ bew-PROH-pee-on am-fa-BEW-teh-moan |
| Trade names | Wellbutrin, Zyban, others |
| Other names | Amfebutamone; 3-Chloro-N-tert-butyl-β-keto-α-methylphenethylamine; 3-Chloro-N-tert-butyl-β-ketoamphetamine; 3-Chloro-N-tert-butylcathinone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695033 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral (swallowed by mouth)[2][3] |
| Drug class | NDRI antidepressants |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown[2] |
| Protein binding | Bupropion: 84%[14] Hydroxybupropion: 77%[14] Threohydrobupropion: 42%[14] |
| Metabolism | Liver, intestines (CYP2B6, others)[2] |
| Metabolites | • Hydroxybupropion • Threohydrobupropion • Erythrohydrobupropion • Others |
| Elimination half-life | Bupropion: alpha: 0.5–1.04 beta: 11 h[15][2] Hydroxybupropion: 20 h[2] Threohydrobupropion: 37 h[2] Erythrohydrobupropion: 33 h[2] |
| Excretion | Urine: 87% (0.5% unchanged)[2] Feces: 10%[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H18ClNO |
| Molar mass | 239.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Bupropion, formerly called amfebutamone,[16] and sold under the brand name Wellbutrin among others, is an atypical antidepressant primarily used to treat major depressive disorder, seasonal affective disorder and to support smoking cessation.[17][18] It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant.[18][19] Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction,[18] it is not associated with weight gain[18] and sleepiness,[20] and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue.[21] Bupropion, particularly the immediate-release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder.[22] The medication is taken by mouth.[2][3]
Common adverse effects of bupropion with the greatest difference from placebo are dry mouth, nausea, constipation, insomnia, anxiety, tremor, and excessive sweating.[10][11] Raised blood pressure is notable.[23] Rare but serious side effects include seizures,[10][11] liver toxicity,[24] psychosis,[25] and risk of overdose.[26] Bupropion use during pregnancy may be associated with increased likelihood of congenital heart defects.[27]
Bupropion acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) and a nicotinic receptor antagonist.[2] However, its effects on dopamine are weak and clinical significance is contentious.[28][29][30][31][32] Chemically, bupropion is an aminoketone that belongs to the class of substituted cathinones and more generally that of substituted amphetamines and substituted phenethylamines.[33][34]
Bupropion was invented by Nariman Mehta, who worked at Burroughs Wellcome, in 1969.[35] It was first approved for medical use in the United States in 1985.[36] Bupropion was originally called by the generic name amfebutamone, before being renamed in 2000.[16] In 2022, it was the 21st most commonly prescribed medication in the United States, with more than 25 million prescriptions.[37][38] It is on the World Health Organization's List of Essential Medicines.[39]In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropion to serve as a rapid-acting antidepressant in patients with major depressive disorder.[40]
- ^ "Bupropion Use During Pregnancy". Drugs.com. Archived from the original on 24 December 2018. Retrieved 24 December 2018.
- ^ a b c d e f g h i j k Cite error: The named reference
pmid31124380was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
pmid16027765was invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "TGA eBS – Product and Consumer Medicine Information Licence". Archived from the original on 7 December 2016. Retrieved 9 January 2023.
- ^ Brazilian Health Regulatory Agency (Anvisa) (31 March 2023). "RDC Nº 784 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ "Wellbutrin Product information". Health Canada. 25 April 2012. Archived from the original on 9 January 2023. Retrieved 9 January 2023.
- ^ "Zyban Product information". Health Canada. 25 April 2012. Archived from the original on 9 January 2023. Retrieved 9 January 2023.
- ^ "Zyban 150 mg prolonged release tablets – Summary of Product Characteristics (SmPC)". (emc). 21 April 2022. Archived from the original on 9 January 2023. Retrieved 9 January 2023.
- ^ a b c Cite error: The named reference
Wellbutrin SR FDA labelwas invoked but never defined (see the help page). - ^ a b c "Wellbutrin XL- bupropion hydrochloride tablet, extended release". DailyMed. 4 March 2022. Archived from the original on 9 January 2023. Retrieved 9 January 2023.
- ^ Cite error: The named reference
Aplenzin FDA labelwas invoked but never defined (see the help page). - ^ "Bupropion hydrochloride EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 18 August 2022. Retrieved 7 March 2023.
- ^ a b c "Zyban 150 mg prolonged release film-coated tablets – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. GlaxoSmithKline UK. 1 August 2013. Archived from the original on 20 July 2017. Retrieved 22 October 2013.
- ^ Cite error: The named reference
pmid27255113was invoked but never defined (see the help page). - ^ a b World Health Organization (2000). "International nonproprietary names for pharmaceutical substances (INN) : proposed international nonproprietary names : list 83". WHO Drug Information. 14 (2). hdl:10665/58135.
- ^ Sweetman S (2011). Martindale: The Complete Drug Reference (37th ed.). Pharmaceutical Press. p. 402. ISBN 978-0-85369-982-8.
- ^ a b c d Cite error: The named reference
pmid27141292was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid27591914was invoked but never defined (see the help page). - ^ Cite error: The named reference
Dhillonwas invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid16848671was invoked but never defined (see the help page). - ^ Steinert T, Fröscher W (July 2018). "Epileptic Seizures Under Antidepressive Drug Treatment: Systematic Review". Pharmacopsychiatry. 51 (4): 121–135. doi:10.1055/s-0043-117962. PMID 28850959. S2CID 22436728.
- ^ Cite error: The named reference
pmid15705013was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid24362450was invoked but never defined (see the help page). - ^ Cite error: The named reference
Buproprion-induced psychosiswas invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid28135844was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid33354752was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid33977870was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid24484978was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid28965364was invoked but never defined (see the help page). - ^ Cite error: The named reference
DeBattista2022was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid35068363was invoked but never defined (see the help page). - ^ "Bupropion". PubChem. United States National Library of Medicine – National Center for Biotechnology Information. 28 July 2018. Archived from the original on 29 July 2018. Retrieved 29 July 2018.
- ^ Dye LR, Murphy C, Calello DP, Levine MD, Skolnik A (2017). Case Studies in Medical Toxicology: From the American College of Medical Toxicology. Springer. p. 85. ISBN 978-3-319-56449-4. Archived from the original on 29 August 2021. Retrieved 5 June 2020.
- ^ Mehta NB (25 June 1974). "United States Patent 3,819,706: Meta-chloro substituted α-butylamino-propiophenones". USPTO. Archived from the original on 7 November 2017. Retrieved 2 June 2008.
- ^ Cite error: The named reference
approvalswas invoked but never defined (see the help page). - ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Bupropion Drug Usage Statistics, United States, 2013–2022". ClinCalc. Retrieved 30 August 2024.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, et al. (March 2021). "Efficacy of dextromethorphan for the treatment of depression: a systematic review of preclinical and clinical trials". Expert Opinion on Emerging Drugs. 26 (1): 63–74. doi:10.1080/14728214.2021.1898588. PMID 33682569. S2CID 232141396.
Previous Page Next Page


