Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Catecholamine
| Catecholamines |
|---|
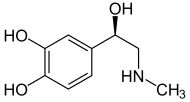 Epinephrine (Adrenaline) |
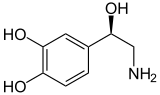 Norepinephrine (Noradrenaline) |
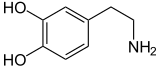 Dopamine |

A catecholamine (/ˌkætəˈkoʊləmiːn/; abbreviated CA) is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine.[1]
Catechol can be either a free molecule or a substituent of a larger molecule, where it represents a 1,2-dihydroxybenzene group.
Catecholamines are derived from the amino acid tyrosine, which is derived from dietary sources as well as synthesis from phenylalanine.[2] Catecholamines are water-soluble and are 50% bound to plasma proteins in circulation.
Included among catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine. Release of the hormones epinephrine and norepinephrine from the adrenal medulla of the adrenal glands is part of the fight-or-flight response.[3]
Tyrosine is created from phenylalanine by hydroxylation by the enzyme phenylalanine hydroxylase. Tyrosine is also ingested directly from dietary protein. Catecholamine-secreting cells use several reactions to convert tyrosine serially to L-DOPA and then to dopamine. Depending on the cell type, dopamine may be further converted to norepinephrine or even further converted to epinephrine.[4]
Various stimulant drugs (such as a number of substituted amphetamines) are catecholamine analogues.
- ^ Fitzgerald, P. A. (2011). "Chapter 11. Adrenal Medulla and Paraganglia". In Gardner, D. G.; Shoback, D. (eds.). Greenspan's Basic & Clinical Endocrinology (9th ed.). New York: McGraw-Hill. Retrieved October 26, 2011.
- ^ Purves, D.; Augustine, G. J.; Fitzpatrick, D.; Hall, W. C.; LaMantia, A. S.; McNamara, J. O.; White, L. E., eds. (2008). Neuroscience (4th ed.). Sinauer Associates. pp. 137–138. ISBN 978-0-87893-697-7.
- ^ "Catecholamines". Health Library. San Diego, CA: University of California. Archived from the original on July 16, 2011.
- ^ Joh, T. H.; Hwang, O. (1987). "Dopamine Beta-Hydroxylase: Biochemistry and Molecular Biology". Annals of the New York Academy of Sciences. 493: 342–350. doi:10.1111/j.1749-6632.1987.tb27217.x. PMID 3473965. S2CID 86229251.
Previous Page Next Page


