Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Chloroform
You can help expand this article with text translated from the corresponding article in Turkish. (September 2024) Click [show] for important translation instructions.
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloromethane | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | R-20, TCM | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.603 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1888 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
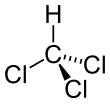
| CHCl3 | |||
| Molar mass | 119.37 g·mol−1 | ||
| Appearance | Highly refractive colorless liquid | ||
| Odor | Sweet, minty, pleasant | ||
| Density | 1.564 g/cm3 (−20 °C) 1.489 g/cm3 (25 °C) 1.394 g/cm3 (60 °C) | ||
| Melting point | −63.5 °C (−82.3 °F; 209.7 K) | ||
| Boiling point | 61.15 °C (142.07 °F; 334.30 K) decomposes at 450 °C | ||
| 10.62 g/L (0 °C) 8.09 g/L (20 °C) 7.32 g/L (60 °C) | |||
| Solubility | Soluble in benzene Miscible in diethyl ether, oils, ligroin, alcohol, CCl4, CS2 | ||
| Solubility in acetone | ≥ 100 g/L (19 °C) | ||
| Solubility in dimethyl sulfoxide | ≥ 100 g/L (19 °C) | ||
| Vapor pressure | 0.62 kPa (−40 °C) 7.89 kPa (0 °C) 25.9 kPa (25 °C) 313 kPa (100 °C) 2.26 MPa (200 °C) | ||
Henry's law
constant (kH) |
3.67 L·atm/mol (24 °C) | ||
| Acidity (pKa) | 15.7 (20 °C) | ||
| UV-vis (λmax) | 250 nm, 260 nm, 280 nm | ||
| −59.30·10−6 cm3/mol | |||
| Thermal conductivity | 0.13 W/(m·K) (20 °C) | ||
Refractive index (nD)
|
1.4459 (20 °C) | ||
| Viscosity | 0.563 cP (20 °C) | ||
| Structure | |||
| Tetrahedral | |||
| 1.15 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
114.25 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
202.9 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−134.3 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
−71.1 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
473.21 kJ/mol | ||
| Pharmacology | |||
| N01AB02 (WHO) | |||
| Hazards[9] | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Decomposes to extremely toxic phosgene and hydrogen chloride in presence of light – possibly carcinogenic – Reproductive toxicity – hepatotoxic[3][4][5] | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302, H315, H319, H331, H336, H351, H361d, H372 | |||
| P201, P202, P235, P260, P264, P270, P271, P280, P281, P301+P330+P331, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P311, P314, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Nonflammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
704 mg/kg (mouse, dermal)[6] | ||
LC50 (median concentration)
|
47,702 mg/m3 (rat, 4 hr)[7] | ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
50 ppm (240 mg/m3)[4] | ||
REL (Recommended)
|
Ca ST 2 ppm (9.78 mg/m3) [60-minute][4] | ||
IDLH (Immediate danger)
|
500 ppm[4][clarification needed] | ||
| Safety data sheet (SDS) | [1] | ||
| Related compounds | |||
Related compounds
|
| ||
| Supplementary data page | |||
| Chloroform (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chloroform,[10] or trichloromethane (often abbreviated as TCM), is an organochloride with the formula CHCl3 and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE.[11] Chloroform was once used as an inhalational anesthetic between the 19th century and the first half of the 20th century.[12][13] It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C).
- ^ Gregory, William, A Handbook of Organic Chemistry (Third edition corrected and much extended), 1852, page 177
- ^ Daniel Pereira Gardner, Medicinal Chemistry for the Use of Students and the Profession: Being a Manual of the Science, with Its Applications to Toxicology, Physiology, Therapeutics, Hygiene, Etc (1848), page 271
- ^ "Part 3 Health Hazards" (PDF). Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Second revised edition. United Nations. Archived (PDF) from the original on 4 March 2019. Retrieved 30 September 2017.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0127". National Institute for Occupational Safety and Health (NIOSH).
- ^ Toxicity on PubChem Archived 17 August 2018 at the Wayback Machine
- ^ Lewis, Richard J. (2012). Sax's Dangerous Properties of Industrial Materials (12th ed.). Wiley. ISBN 978-0-470-62325-1.
- ^ "Chloroform" (PDF). Environmental Protection Agency. September 2016. Retrieved 19 February 2024.
- ^ "Chloroform". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "PubChem: Safety and Hazards – GHS Classification". National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 17 August 2018. Retrieved 17 August 2018.
- ^ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 661. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The retained names 'bromoform' for HCBr3, 'chloroform' for HCCl3, and 'iodoform' for HCI3 are acceptable in general nomenclature. Preferred IUPAC names are substitutive names.
- ^ Rossberg, M.; et al. "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2. ISBN 978-3527306732.
- ^ "Ether and Chloroform". Archived from the original on 24 March 2018. Retrieved 24 April 2018.
- ^ "Chloroform [MAK Value Documentation, 2000]". The MAK-Collection for Occupational Health and Safety. 2012. pp. 20–58. doi:10.1002/3527600418.mb6766e0014. ISBN 978-3-527-60041-0.
Previous Page Next Page