Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
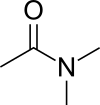
Dimethylacetamide
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylacetamide | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DMA, DMAC, DMAc[1] |
| 1737614 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.389 |
| EC Number |
|
| MeSH | dimethylacetamide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H9NO | |
| Molar mass | 87.122 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Ammoniacal |
| Density | 0.937 g/mL |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 165.1 °C; 329.1 °F; 438.2 K |
| Miscible | |
| log P | −0.253 |
| Vapor pressure | 300 Pa |
| UV-vis (λmax) | 270 nm |
Refractive index (nD)
|
1.4375 |
| Viscosity | 0.945 mPa·s [2] |
| Thermochemistry | |
Heat capacity (C)
|
178.2 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−300.1 kJ/mol |
Std enthalpy of
combustion (ΔcH⦵298) |
−2.5835–−2.5805 MJ/mol |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H312, H319, H332, H360 | |
| P280, P308+P313 | |
| NFPA 704 (fire diamond) | |
| Flash point | 63 °C (145 °F; 336 K) |
| 490 °C (914 °F; 763 K) | |
| Explosive limits | 1.8–11.5% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2.24 g/kg (dermal, rabbit) 4.3 g/kg (oral, rat) 4.8 g/kg (oral, rat) 4.62 g/kg (oral, mouse)[4] |
LC50 (median concentration)
|
2475 ppm (rat, 1 h)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 10 ppm (35 mg/m3) [skin][3] |
REL (Recommended)
|
TWA 10 ppm (35 mg/m3) [skin][3] |
IDLH (Immediate danger)
|
300 ppm[3] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.
- ^ Munro, D. D.; Stoughton, R. B. (1965). "Dimethylacetamide (DMAC) and Dimethylformamide (DMFA). Effect on Percutaneous Absorption". Archives of Dermatology. 92 (5): 585–586. doi:10.1001/archderm.1965.01600170101020. PMID 5844405.
- ^ Iloukhani, H., K. Khanlarzadeh. "Densities, viscosities, and refractive indices for binary and ternary mixtures of N, N-dimethylacetamide (1)+ 2-methylbutan-2-ol (2)+ ethyl acetate (3) at 298.15 K for m liquid region and at ambient pressure". Journal of Chemical & Engineering Data, 51.4 (2006): 1226–1231. doi:10.1021/je050538q.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0218". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Dimethyl acetamide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
Previous Page Next Page



