Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
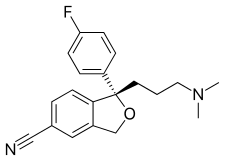
Escitalopram
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛsəˈtæləˌpræm/ ⓘ |
| Trade names | Cipralex, Lexapro, others[1] |
| Other names | (S)-Citalopram; S-Citalopram; S-(+)-Citalopram; S(+)-Citalopram; (+)-Citalopram; LU-26054; MLD-55 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603005 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Selective serotonin reuptake inhibitor (SSRI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~80%[7][8] |
| Protein binding | ~55–56% (low)[7][8] |
| Metabolism | Liver (CYP2C19, CYP3A4, CYP2D6)[7][8] |
| Metabolites | • Desmethylcitalopram[7][8] • Didesmethylcitalopram[7][8] |
| Elimination half-life | ~27–32 hours[7] |
| Excretion | Urine (major; 8–10% unchanged), feces (minor)[8] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.244.188 |
| Chemical and physical data | |
| Formula | C20H21FN2O |
| Molar mass | 324.399 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Levorotatory enantiomer |
| |
| |
| (verify) | |
Escitalopram, sold under the brand names Lexapro and Cipralex, among others, is an antidepressant medication of the selective serotonin reuptake inhibitor (SSRI) class,[9] which is mainly used to treat major depressive disorder and generalized anxiety disorder.[9] It is taken by mouth,[9] and available commercially as an oxalate salt exclusively.
Common side effects include headache, nausea, sexual problems, mild sedation, and trouble sleeping.[9] More serious side effects may include suicidal thoughts in people up to the age of 24 years.[9] It is unclear if use during pregnancy or breastfeeding is safe.[10] Escitalopram is the (S)-enantiomer of citalopram (which exists as a racemate), hence the name es-citalopram.[9]
Escitalopram was approved for medical use in the United States in 2002.[9] Escitalopram is rarely replaced by twice the dose of citalopram; escitalopram is safer and more effective.[11] It is on the World Health Organization's List of Essential Medicines.[12] In 2022, it was the second most prescribed antidepressant and fifteenth most commonly prescribed medication in the United States, with more than 30 million prescriptions.[13][14] In Australia, it was one of the top 10 most prescribed medications between 2017 and 2023.[15]
The drug is often found to have superior efficacy to other antidepressants in alleviating the symptoms of depression and anxiety, followed by another SSRI, sertraline, but escitalopram is less likely to trigger diarrhea and nausea. Other first-line SSRIs that have similar but slightly less potency include paroxetine and fluoxetine—the latter is also commonly associated with a gastrointestinal side effect profile.
- ^ Cite error: The named reference
drugsINTwas invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ "Lexapro- escitalopram tablet, film coated; Lexapro- escitalopram solution". DailyMed. 17 November 2023. Retrieved 31 December 2023.
- ^ Human Medicines Division (September 2022). "Active substance(s): escitalopram" (PDF). List of nationally authorised medicinal products. European Medicines Agency. Archived (PDF) from the original on 6 September 2022. Retrieved 6 September 2022.
- ^ a b c d e f Pastoor D, Gobburu J (January 2014). "Clinical pharmacology review of escitalopram for the treatment of depression". Expert Opin Drug Metab Toxicol. 10 (1): 121–128. doi:10.1517/17425255.2014.863873. PMID 24289655.
- ^ a b c d e f Rao N (2007). "The clinical pharmacokinetics of escitalopram". Clin Pharmacokinet. 46 (4): 281–290. doi:10.2165/00003088-200746040-00002. PMID 17375980.
- ^ a b c d e f g "X". The American Society of Health-System Pharmacists. Archived from the original on 29 December 2017. Retrieved 28 December 2017.
- ^ "Escitalopram (Lexapro) Use During Pregnancy". Drugs.com. Archived from the original on 31 December 2017. Retrieved 31 December 2017.
- ^ "Protocol for switching patients from escitalopram to citalopram". NHS. 2015. Archived from the original on 10 August 2020. Retrieved 13 February 2018.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Escitalopram Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Medicines in the health system". Australian Institute of Health and Welfare. 2 July 2024. Retrieved 30 September 2024.
Previous Page Next Page


