Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Fayalite
| Fayalite | |
|---|---|
 Fayalite crystals on substrate. Sample collected from Ochtendung, Eifel, Germany | |
| General | |
| Category | Nesosilicate |
| Formula (repeating unit) | Fe2SiO4 |
| IMA symbol | Fa[1] |
| Strunz classification | 9.AC.05 |
| Crystal system | Orthorhombic |
| Crystal class | Dipyramidal (mmm) H-M symbol: (2/m 2/m 2/m) |
| Space group | Pbnm (no. 62) |
| Unit cell | a = 4.8211, b = 10.4779 c = 6.0889 [Å]; Z = 4 |
| Identification | |
| Formula mass | 203.771 g·mol−1 |
| Color | Greenish yellow, yellow-brown, brown; pale yellow to amber in thin section |
| Crystal habit | Commonly granular, compact, or massive |
| Twinning | On [100]; also on [031], as trillings |
| Cleavage | {010} moderate, {100} imperfect |
| Fracture | Conchoidal |
| Mohs scale hardness | 6.5 – 7.0 |
| Luster | Vitreous to resinous on fractures |
| Streak | White |
| Diaphaneity | Transparent |
| Specific gravity | 4.392 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.731 – 1.824 nβ = 1.760 – 1.864 nγ = 1.773 – 1.875 |
| Birefringence | δ = 0.042 – 0.051 |
| Pleochroism | Faint |
| 2V angle | Measured: 74° to 47°, Calculated: 54° to 66° |
| References | [2][3][4] |
Fayalite (Fe
2SiO
4, commonly abbreviated to Fa) is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.82 Å, b 10.48 Å and c 6.09 Å.
Fayalite forms solid solution series with the magnesium olivine endmember forsterite (Mg2SiO4) and also with the manganese rich olivine endmember tephroite (Mn2SiO4).
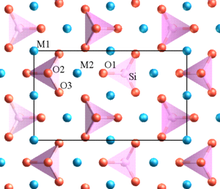
Iron rich olivine is a relatively common constituent of acidic and alkaline igneous rocks such as volcanic obsidians, rhyolites, trachytes and phonolites and plutonic quartz syenites where it is associated with amphiboles. Its main occurrence is in ultramafic volcanic and plutonic rocks and less commonly in felsic plutonic rocks and rarely in granite pegmatite. It also occurs in lithophysae in obsidian. It also occurs in medium-grade thermally metamorphosed iron-rich sediments and in impure carbonate rocks.[2]
Fayalite is stable with quartz at low pressures, whereas more magnesian olivine is not, because of the reaction olivine + quartz = orthopyroxene. Iron stabilizes the olivine + quartz pair. The pressure and compositional dependence of the reaction can be used to calculate constraints on pressures at which assemblages of olivine + quartz formed.
Fayalite can also react with oxygen to produce magnetite + quartz: the three minerals together make up the "FMQ" oxygen buffer. The reaction is used to control the fugacity of oxygen in laboratory experiments. It can also be used to calculate the fugacity of oxygen recorded by mineral assemblages in metamorphic and igneous processes.

At high pressure, fayalite undergoes a phase transition to ahrensite, the iron-bearing analogue of ringwoodite, i.e., contrary to forsterite there is no intermediate form analogous to wadsleyite; under the conditions prevailing in the upper mantle of the Earth, the transition would occur at ca. 6–7 GPa, i.e., at substantially lower pressure than the phase transitions of forsterite.[5] In high-pressure experiments, the transformation may be delayed, so that it may remain stable to pressures of almost 35 GPa (see fig.), at which point it may become amorphous rather than take on a crystalline structure such as ahrensite.
The name fayalite is derived from Faial (Fayal) Island in the Azores where it was first described in 1840.[3]
- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ a b http://rruff.geo.arizona.edu/doclib/hom/fayalite.pdf Handbook of Mineralogy
- ^ a b http://www.mindat.org/min-1458.html Mindat.org
- ^ Fayalite data on Webmineral
- ^ D. C. Presnall (1995): Phase diagrams of Earth-forming minerals. In: Mineral Physics & Crystallography – A Handbook of Physical Constants, ed. by T. J. Ahrens, AGU Reference Shelf vol. 2, American Geophysical Union, Washington, D.C., pp. 248–268
Previous Page Next Page


