Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Formate

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Formate | |||
| Systematic IUPAC name
Methanoate | |||
| Other names
Formylate
Methylate Isocarbonite Carbonite(1-) Hydrogencarboxylate Metacarbonoate Oxocarbinate Oxomethyl oxide ion Oxomethoxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| MeSH | Formates | ||
PubChem CID
|
|||
| UNII | |||
| |||
| Properties | |||
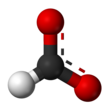
| HCOO− or HCO− 2 | |||
| Molar mass | 45.017 g mol−1 | ||
| Conjugate acid | Formic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion (HCO−2) or its derivatives such as ester of formic acid. The salts and esters are generally colorless.[1]
- ^ Reutemann, Werner; Kieczka, Heinz (2000), "Formic Acid", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a12_013, ISBN 3-527-30673-0
Previous Page Next Page