Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Hydrogen bond
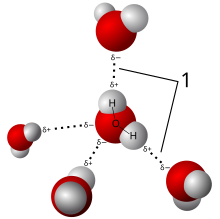

In chemistry, a hydrogen bond (or H-bond) is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond.[5] The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).
Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule).[6][7][8][9] The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol.[10] This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins. Hydrogen bonds are responsible for holding materials such as paper and felted wool together, and for causing separate sheets of paper to stick together after becoming wet and subsequently drying.
The hydrogen bond is also responsible for many of the physical and chemical properties of compounds of N, O, and F that seem unusual compared with other similar structures. In particular, intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group-16 hydrides that have much weaker hydrogen bonds.[11] Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids.
- ^ Sweetman, A. M.; Jarvis, S. P.; Sang, Hongqian; Lekkas, I.; Rahe, P.; Wang, Yu; Wang, Jianbo; Champness, N.R.; Kantorovich, L.; Moriarty, P. (2014). "Mapping the force field of a hydrogen-bonded assembly". Nature Communications. 5: 3931. Bibcode:2014NatCo...5.3931S. doi:10.1038/ncomms4931. PMC 4050271. PMID 24875276.
- ^ Hapala, Prokop; Kichin, Georgy; Wagner, Christian; Tautz, F. Stefan; Temirov, Ruslan; Jelínek, Pavel (2014-08-19). "Mechanism of high-resolution STM/AFM imaging with functionalized tips". Physical Review B. 90 (8): 085421. arXiv:1406.3562. Bibcode:2014PhRvB..90h5421H. doi:10.1103/PhysRevB.90.085421. S2CID 53610973.
- ^ De Luca, S.; Chen, F.; Seal, P.; Stenzel, M. H.; Smith, S. C. (2017). "Binding and Release between Polymeric Carrier and Protein Drug: pH-Mediated Interplay of Coulomb Forces, Hydrogen Bonding, van der Waals Interactions, and Entropy". Biomacromolecules. 18 (11): 3665–3677. doi:10.1021/acs.biomac.7b00657. hdl:1959.4/unsworks_55160. PMID 28880549.
- ^ Hämäläinen, Sampsa K.; van der Heijden, Nadine; van der Lit, Joost; den Hartog, Stephan; Liljeroth, Peter; Swart, Ingmar (2014-10-31). "Intermolecular Contrast in Atomic Force Microscopy Images without Intermolecular Bonds". Physical Review Letters. 113 (18): 186102. arXiv:1410.1933. Bibcode:2014PhRvL.113r6102H. doi:10.1103/PhysRevLett.113.186102. hdl:1874/307996. PMID 25396382. S2CID 8309018. Archived from the original on 2018-01-20. Retrieved 2017-08-30.
- ^ Arunan, Elangannan; Desiraju, Gautam R.; Klein, Roger A.; Sadlej, Joanna; Scheiner, Steve; Alkorta, Ibon; Clary, David C.; Crabtree, Robert H.; Dannenberg, Joseph J. (2011-07-08). "Definition of the hydrogen bond (IUPAC Recommendations 2011)". Pure and Applied Chemistry. 83 (8): 1637–1641. doi:10.1351/PAC-REC-10-01-02. ISSN 1365-3075. S2CID 97688573.
- ^ Pimentel, G. The Hydrogen Bond Franklin Classics, 2018), ISBN 0343171600
- ^ Jeffrey, G. A.; An introduction to hydrogen bonding; Oxford university press New York, 1997. ISBN 0195095499
- ^ Jeffrey, G. A.; Saenger, W. Hydrogen bonding in biological structures; Springer: Berlin, 1994, 2012 Springer; ISBN 3540579036
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "hydrogen bond". doi:10.1351/goldbook.H02899
- ^ Steiner, Thomas (2002). "The Hydrogen Bond in the Solid State". Angew. Chem. Int. Ed. 41 (1): 48–76. doi:10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U. PMID 12491444.
- ^ Sabin, John R. (1971). "Hydrogen bonds involving sulfur. I. Hydrogen sulfide dimer". J. Am. Chem. Soc. 93 (15): 3613–3620. doi:10.1021/ja00744a012.
Previous Page Next Page


