Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
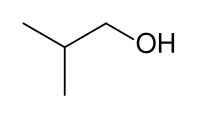
Isobutanol
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpropan-1-ol | |
| Other names
Isobutyl alcohol
IBA 2-Methyl-1-propanol 2-Methylpropyl alcohol Isopropylcarbinol | |
| Identifiers | |
3D model (JSmol)
|
|
| 1730878 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.044 |
| EC Number |
|
| 49282 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1212 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[3] | |
| C4H10O | |
| Molar mass | 74.122 g/mol |
| Appearance | Colorless liquid |
| Odor | sweet, musty[1] |
| Density | 0.802 g/cm3, liquid |
| Melting point | −108 °C (−162 °F; 165 K) |
| Boiling point | 107.89 °C (226.20 °F; 381.04 K) |
| 8.7 mL/100 mL[2] | |
| log P | 0.8 |
| Vapor pressure | 9 mmHg (20°C)[1] |
Refractive index (nD)
|
1.3959 |
| Viscosity | 3.95 cP at 20 °C |
| Hazards[3] | |
| GHS labelling: | |
 
| |
| Danger | |
| H226, H315, H318, H335, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 28 °C (82 °F; 301 K) |
| 415 °C (779 °F; 688 K) | |
| Explosive limits | 1.7–10.9% |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
3750 mg/kg (rabbit, oral) 2460 mg/kg (rat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (300 mg/m3)[1] |
REL (Recommended)
|
TWA 50 ppm (150 mg/m3)[1] |
IDLH (Immediate danger)
|
1600 ppm[1] |
| Safety data sheet (SDS) | ICSC 0113 |
| Related compounds | |
Related butanols
|
1-Butanol sec-Butanol tert-Butanol |
Related compounds
|
Isobutyraldehyde Isobutyric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.[5]
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0352". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Iso-butanol". ChemicalLand21.
- ^ a b Isobutanol, International Chemical Safety Card 0113, Geneva: International Programme on Chemical Safety, April 2005.
- ^ "Isobutyl alcohol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page).
Previous Page Next Page



