Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Methane
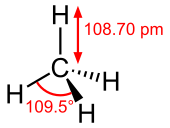
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methane[1] | |||
| Systematic IUPAC name
Carbane (never recommended[1]) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1718732 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.739 | ||
| EC Number |
| ||
| 59 | |||
| KEGG | |||
| MeSH | Methane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1971 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH4 | |||
| Molar mass | 16.043 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | |||
| Melting point | −182.456 °C (−296.421 °F; 90.694 K)[3] | ||
| Boiling point | −161.49 °C (−258.68 °F; 111.66 K)[6] | ||
| Critical point (T, P) | 190.56 K (−82.59 °C; −116.66 °F), 4.5992 MPa (45.391 atm) | ||
| 22.7 mg/L[4] | |||
| Solubility | Soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone and insoluble in water | ||
| log P | 1.09 | ||
Henry's law
constant (kH) |
14 nmol/(Pa·kg) | ||
| Conjugate acid | Methanium | ||
| Conjugate base | Methyl anion | ||
| −17.4×10−6 cm3/mol[5] | |||
| Structure | |||
| Td | |||
| Tetrahedral at carbon atom | |||
| 0 D | |||
| Thermochemistry[7] | |||
Heat capacity (C)
|
35.7 J/(K·mol) | ||
Std molar
entropy (S⦵298) |
186.3 J/(K·mol) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−74.6 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
−50.5 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−891 kJ/mol | ||
| Hazards[8] | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −188 °C (−306.4 °F; 85.1 K) | ||
| 537 °C (999 °F; 810 K) | |||
| Explosive limits | 4.4–17% | ||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Methane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methane (US: /ˈmɛθeɪn/ METH-ayn, UK: /ˈmiːθeɪn/ MEE-thayn) is a chemical compound with the chemical formula CH4 (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an organic compound, and among the simplest of organic compounds. Methane is also a hydrocarbon.
Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane.[10]
The Earth's atmospheric methane concentration has increased by about 160% since 1750, with the overwhelming percentage caused by human activity.[11] It accounted for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases, according to the 2021 Intergovernmental Panel on Climate Change report.[12] Strong, rapid and sustained reductions in methane emissions could limit near-term warming and improve air quality by reducing global surface ozone.[13]
Methane has also been detected on other planets, including Mars, which has implications for astrobiology research.[14]
- ^ a b "General Principles, Rules, and Conventions". Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. P-12.1. doi:10.1039/9781849733069-00001. ISBN 978-0-85404-182-4.
Methane is a retained name (see P-12.3) that is preferred to the systematic name 'carbane', a name never recommended to replace methane, but used to derive the names 'carbene' and 'carbyne' for the radicals H2C2• and HC3•, respectively.
- ^ "Gas Encyclopedia". Archived from the original on December 26, 2018. Retrieved November 7, 2013.
- ^ a b Haynes, p. 3.344
- ^ Haynes, p. 5.156
- ^ Haynes, p. 3.578
- ^ CRC Handbook of Chemistry and Physics, 49th edition
- ^ Haynes, pp. 5.26, 5.67
- ^ "Safety Datasheet, Material Name: Methane" (PDF). US: Metheson Tri-Gas Incorporated. December 4, 2009. Archived from the original (PDF) on June 4, 2012. Retrieved December 4, 2011.
- ^ NOAA Office of Response and Restoration, US GOV. "METHANE". noaa.gov. Archived from the original on January 9, 2019. Retrieved March 20, 2015.
- ^ Khalil, M. A. K. (1999). "Non-Co2 Greenhouse Gases in the Atmosphere". Annual Review of Energy and the Environment. 24: 645–661. doi:10.1146/annurev.energy.24.1.645.
- ^ Global Methane Assessment (PDF). United Nations Environment Programme and Climate and Clean Air Coalition (Report). Nairobi. 2022. p. 12. Retrieved March 15, 2023.
- ^ "Climate Change 2021. The Physical Science Basis. Summary for Policymakers. Working Group I contribution to the WGI Sixth Assessment Report of the Intergovernmental Panel on Climate Change". IPCC. The Intergovernmental Panel on Climate Change. Archived from the original on August 22, 2021. Retrieved August 22, 2021.
- ^ IPCC, 2023: Summary for Policymakers. In: Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)]. IPCC, Geneva, Switzerland, page 26, section C.2.3
- ^ Etiope, Giuseppe; Lollar, Barbara Sherwood (2013). "Abiotic Methane on Earth". Reviews of Geophysics. 51 (2): 276–299. Bibcode:2013RvGeo..51..276E. doi:10.1002/rog.20011. S2CID 56457317.
Previous Page Next Page





