Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Methylmagnesium chloride
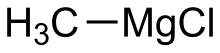 | |
| Names | |
|---|---|
| IUPAC name
chlorido(methyl)magnesium
| |
| Other names
(chloromagnesio)methane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.573 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3MgCl | |
| Molar mass | 74.79 g/mol |
| Appearance | colorless solid |
| Reacts with water | |
| Solubility | soluble in diethyl ether and THF |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable, Reacts with water |
| GHS labelling: | |
 
| |
| Danger | |
| H225, H250, H260, H314 | |
| P210, P222, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P402+P404, P403+P235, P405, P422, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −17 °C (1 °F; 256 K) |
| Related compounds | |
Related compounds
|
Phenylmagnesium bromide, Dibutylmagnesium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methylmagnesium chloride is an organometallic compound with the general formula CH3MgCl. This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran.
Previous Page Next Page



