Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Neodymium(III) carbonate
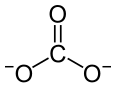
| |
| Names | |
|---|---|
| IUPAC name
neodymium(3+);tricarbonate
| |
| Other names
neodymium(III) carbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.025.072 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Nd2(CO3)3 | |
| Molar mass | 468.53 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P321, P362+P364, P403+P233, P405, P501 | |
| Related compounds | |
Other anions
|
neodymium(III) oxide, neodymium(III) hydroxide |
Other cations
|
praseodymium(III) carbonate samarium(III) carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion has charge -2.[1] It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red,[2] while the octahydrate is a pink solid.[3] Both of these salts are insoluble in water.[4]
- ^ See https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium_III_-carbonate-hydrate
- ^ Rare earth elements: Main volume, Phần 3 (Leopold Gmelin; Verlag Chemie, 1994), page 22; 68. Retrieved 4 February 2021.
- ^ Handbook… (Pierre Villars, Karin Cenzual, Roman Gladyshevskii; Walter de Gruyter GmbH & Co KG, 24 thg 7, 2017 - 1970 pages), page 999. Retrieved 4 February 2021.
- ^ Cite error: The named reference
wjwas invoked but never defined (see the help page).
Previous Page Next Page


