Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
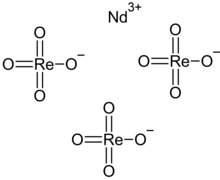
Neodymium perrhenate
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| |
| Properties | |
| Nd(ReO4)3 | |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Neodymium perrhenate is an inorganic compound with the chemical formula Nd(ReO4)3, which exists in anhydrous and tetrahydrate.[1][2] It can be obtained by reacting excess neodymium oxide with 240 g/L perrhenic acid solution.[3] In its solution, NdReO42+ and Nd(ReO4)2+ can be observed with stability constants of 16.5 and 23.6, respectively.[4]
Nd4Re6O19 can be obtained by reacting neodymium perrhenate and NdRe2 at high temperature.[5]
- ^ Varfolomeev, M. B.; Plyushchev, V. E.; Rybakov, K. A. Monoclinic modification of neodymium perrhenate tetrahydrate. Zhurnal Neorganicheskoi Khimii, 1967. 12 (5): 1410-1411. ISSN 0044-457X.
- ^ Mashonkin, V. P.; Ilyukhin, V. V.; Varfolomeev, M. B. Structure of rhombic rare earth perrhenate tetrahydrates RE(ReO4)·3.4H2O (RE = praseodymium-·dysprosium). Koordinatsionnaya Khimiya, 1977. 3 (7): 1014-1019. ISSN 0132-344X.
- ^ Plyushchev, V. E.; Amosov, V. M.; Varfolomeev, M. B. Synthesis and certain properties of lower crystallohydrates of yttrium, lanthanum, and lanthanoid perrhenates. Doklady Akademii Nauk SSSR, 1963. 150 (1): 105-108. ISSN 0002-3264.
- ^ Petrov, K. I.; Orlin, N. A.; Plyushchev, V. E. Neodymium perrhenate complexes studied by a spectrographic method. Zhurnal Neorganicheskoi Khimii, 1969. 14 (10): 2739-2742. ISSN 0044-457X.
- ^ Wolfgang Jeitschko, Dieter H Heumannskämper, Marietta S Schriewer-Pöttgen, Ute Ch Rodewald (October 1999). "Preparation, Crystal Structures, and Properties of Rhenates with Multiple Re–Re Bonds: Ln2ReO5 (Ln=Sm, Eu, Gd), Ln3Re2O9 (Ln=Pr, Nd, Sm), and Ln4Re6O19 (Ln=La–Nd)". Journal of Solid State Chemistry. 147 (1): 218–228. Bibcode:1999JSSCh.147..218J. doi:10.1006/jssc.1999.8237. Archived from the original on 2018-06-08. Retrieved 2020-08-30.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Previous Page Next Page


