Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Nitrogen mustard

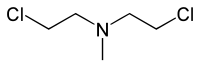
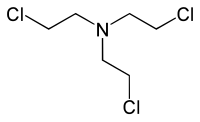
Nitrogen mustards (NMs) are cytotoxic organic compounds with the bis(2-chloroethyl)amino ((ClC2H4)2NR) functional group.[1] Although originally produced as chemical warfare agents,[2][3] they were the first chemotherapeutic agents for treatment of cancer.[4] Nitrogen mustards are nonspecific DNA alkylating agents.
- ^ "Mustards". The IUPAC Compendium of Chemical Terminology. 2014. doi:10.1351/goldbook.M04071.
- ^ Nitrogen mustard gas was stockpiled by several nations during the Second World War, but it was never used in combat.Daniel C. Keyes; Jonathan L. Burstein; Richard B. Schwartz; Raymond E. Swienton (2004). Medical Response to Terrorism: Preparedness and Clinical Practice. Lippincott Williams & Wilkins. p. 16. ISBN 978-0781749862 – via books.google.com.
- ^ Centers for Disease Control and Prevention (April 4, 2013). "Facts About Nitrogen Mustards". cdc.gov. Archived from the original on September 3, 2013. Retrieved September 12, 2013.
- ^ Chabner, Bruce A.; Roberts, Thomas G. (2005). "Chemotherapy and the war on cancer". Nature Reviews Cancer. 5 (1): 65–72. doi:10.1038/nrc1529. PMID 15630416. S2CID 205467419.
Previous Page Next Page


