Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
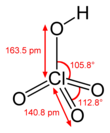
Perchloric acid
| |||
 | |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Perchloric acid | |||
| Other names
Hyperchloric acid[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.648 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1873 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| HClO4 | |||
| Molar mass | 100.46 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | odorless | ||
| Density | 1.768 g/cm3 | ||
| Melting point | −17 °C (1 °F; 256 K) (72% aqueous solution)[2] −112 °C (anhydrous) | ||
| Boiling point | 203 °C (397 °F; 476 K) (azeotrope)[2] | ||
| Miscible | |||
| Acidity (pKa) | −15.2 (±2.0);[3] ≈ −10 | ||
| Conjugate base | Perchlorate | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Powerful oxidizer, highly corrosive | ||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H271, H290, H302, H314, H373 | |||
| P210, P280, P303+P361+P353, P304+P340, P305+P351+P338, P310, P371, P375, P380 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | ICSC 1006 | ||
| Related compounds | |||
Related compounds
|
Hydrochloric acid Hypochlorous acid Chlorous acid Chloric acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Perchloric acid is a mineral acid with the formula HClO4. It is an oxoacid of chlorine. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.[4]
- ^ Fomon, S. (1920). Medicine and the Allied Sciences. D. Appleton. p. 148.
- ^ a b "Safety (MSDS) data for perchloric acid, 70%". msds.chem.ox.ac.uk. 2 July 2008. Archived from the original on 2 July 2008. Retrieved 24 February 2022.
- ^ Trummal, Aleksander; Lipping, Lauri; Kaljurand, Ivari; Koppel, Ilmar A.; Leito, Ivo (6 May 2016). "Acidity of Strong Acids in Water and Dimethyl Sulfoxide". The Journal of Physical Chemistry A. 120 (20). American Chemical Society (ACS): 3663–3669. Bibcode:2016JPCA..120.3663T. doi:10.1021/acs.jpca.6b02253. ISSN 1089-5639. PMID 27115918. S2CID 29697201.
- ^ "Perchloric Acid | Environmental Health & Safety | Michigan State University". ehs.msu.edu. Retrieved 2023-11-02.
Previous Page Next Page