Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Periodic trends
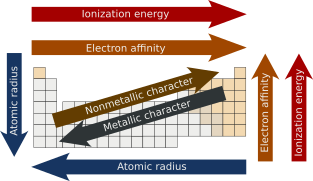
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain elements when grouped by period and/or group. They were discovered by the Russian chemist Dmitri Mendeleev in 1863. Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, nucleophilicity, electrophilicity, valency, nuclear charge, and metallic character.[1] Mendeleev built the foundation of the periodic table.[2] Mendeleev organized the elements based on atomic weight, leaving empty spaces where he believed undiscovered elements would take their places.[3] Mendeleev’s discovery of this trend allowed him to predict the existence and properties of three unknown elements, which were later discovered by other chemists and named gallium, scandium, and germanium.[4] English physicist Henry Moseley discovered that organizing the elements by atomic number instead of atomic weight would naturally group elements with similar properties.[3]
- ^ Schrobilgen, Gary J. (2019). "Chemistry at the Edge of the Periodic Table: The Importance of Periodic Trends on the Discovery of the Noble Gases and the Development of Noble-Gas Chemistry". In Mingos, D. Michael P. (ed.). The Periodic Table I. Structure and Bonding. Vol. 181. pp. 157–196. doi:10.1007/430_2019_49. ISBN 978-3-030-40024-8.
- ^ Edwards, Peter P.; Egdell, Russell G.; Fenske, Dieter; Yao, Benzhen (18 September 2020). "The periodic law of the chemical elements: 'The new system of atomic weights which renders evident the analogies which exist between bodies'". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 378 (2180): 20190537. Bibcode:2020RSPTA.37890537E. doi:10.1098/rsta.2019.0537. PMC 7435142. PMID 32811357.
- ^ a b Egdell, Russell G.; Bruton, Elizabeth (2020-09-18). "Henry Moseley, X-ray spectroscopy and the periodic table". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 378 (2180): 20190302. Bibcode:2020RSPTA.37890302E. doi:10.1098/rsta.2019.0302. PMID 32811359.
- ^ Sztejnberg, Aleksander (2018). "Dmitri Ivanovich Mendeleev (1834 – 1907), Prominent Russian Scientist. References to His Great Scientific Achievements in the Literature between 1871 and 1917". Revista CENIC. Ciencias Químicas. 49 (1): 1–13.
Previous Page Next Page


