Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Photoelectric effect
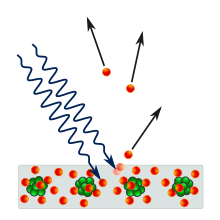
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.
The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the light's intensity or duration of exposure. Because a low-frequency beam at a high intensity does not build up the energy required to produce photoelectrons, as would be the case if light's energy accumulated over time from a continuous wave, Albert Einstein proposed that a beam of light is not a wave propagating through space, but a swarm of discrete energy packets, known as photons—term coined by Gilbert N. Lewis in 1926.[1]
Emission of conduction electrons from typical metals requires a few electron-volt (eV) light quanta, corresponding to short-wavelength visible or ultraviolet light. In extreme cases, emissions are induced with photons approaching zero energy, like in systems with negative electron affinity and the emission from excited states, or a few hundred keV photons for core electrons in elements with a high atomic number.[2] Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality.[3] Other phenomena where light affects the movement of electric charges include the photoconductive effect, the photovoltaic effect, and the photoelectrochemical effect.
- ^ December 18, 1926: Gilbert Lewis coins "photon" in letter to Nature.
- ^ "X-Ray Data Booklet". xdb.lbl.gov. Retrieved 2020-06-20.
- ^ Serway, R. A. (1990). Physics for Scientists & Engineers (3rd ed.). Saunders. p. 1150. ISBN 0-03-030258-7.
Previous Page Next Page


