Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
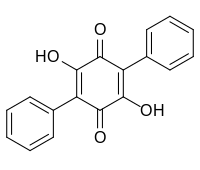
Polyporic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
23,26-Dihydroxy[11,21:24,31-terphenyl]-22,25-dione | |
| Other names
Polyporin; Orygameic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | C118527 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H12O4 | |
| Molar mass | 292.290 g·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polyporic acid is a para-terphenyl benzoquinone compound first identified by German chemist Stahlschmidt from a mycelial culture of the fungus species Hapalopilus nidulans in 1877.[1][2] This chemical, present at 20–40% of the fresh weight of the fruit bodies,[3] inhibits the enzyme dihydroorotate dehydrogenase.[4] It is found in other mushrooms, but in much lower amounts.[4]
In animal studies, consumption of polyporic acid caused reduced locomotor activity, depressed visual placing response, hepatorenal failure, metabolic acidosis, hypokalaemia, and hypocalcaemia.[4] Because these effects are similar to those observed in individuals poisoned by H. nidulans, polyporic acid is thought to be the primary toxin in H. nidulans.[4]
Polyporic acid has some antifungal[5] and antibacterial activity.[6] It has been shown to be an intermediate in the biosynthesis of allantofuranone, a gamma-lactone antibiotic from the fungus Allantophomopsis lycopodina.[7]
- ^ Cite error: The named reference
Stahlschmidt 1877was invoked but never defined (see the help page). - ^ Cite error: The named reference
Calì 2003was invoked but never defined (see the help page). - ^ Cite error: The named reference
Bechtold 2009was invoked but never defined (see the help page). - ^ a b c d Cite error: The named reference
Kraft 1998was invoked but never defined (see the help page). - ^ Cite error: The named reference
Brewer 1977was invoked but never defined (see the help page). - ^ Cite error: The named reference
Brewer 1984was invoked but never defined (see the help page). - ^ Cite error: The named reference
Schüffler 2011was invoked but never defined (see the help page).
Previous Page Next Page


