Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Propane
It has been suggested that parts of this page be moved into Liquefied petroleum gas. (Discuss) (August 2024) |
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propane[1] | |||
| Systematic IUPAC name
Tricarbane (never recommended[1]) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730718 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.753 | ||
| EC Number |
| ||
| E number | E944 (glazing agents, ...) | ||
| 25044 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1978 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[3] | |||
| C3H8 | |||
| Molar mass | 44.097 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | 2.0098 kg/m3 (at 0 °C, 101.3 kPa) | ||
| Melting point | −187.7 °C; −305.8 °F; 85.5 K | ||
| Boiling point | −42.25 to −42.04 °C; −44.05 to −43.67 °F; 230.90 to 231.11 K | ||
| Critical point (T, P) | 370 K (97 °C; 206 °F), 4.23 MPa (41.7 atm) | ||
| 47 mg⋅L−1 (at 0 °C) | |||
| log P | 2.236 | ||
| Vapor pressure | 853.16 kPa (at 21.1 °C (70.0 °F)) | ||
Henry's law
constant (kH) |
15 nmol⋅Pa−1⋅kg−1 | ||
| Conjugate acid | Propanium | ||
| −40.5 × 10−6 cm3/mol | |||
| 0.083 D[2] | |||
| Thermochemistry | |||
Heat capacity (C)
|
73.60 J⋅K−1⋅mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−105.2–104.2 kJ⋅mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.2197–2.2187 MJ⋅mol−1 | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −104 °C (−155 °F; 169 K) | ||
| 470 °C (878 °F; 743 K) | |||
| Explosive limits | 2.37–9.5% | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1,000 ppm (1,800 mg/m3)[4] | ||
REL (Recommended)
|
TWA 1,000 ppm (1,800 mg/m3)[4] | ||
IDLH (Immediate danger)
|
2,100 ppm[4] | ||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Propane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

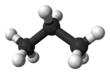
Propane (/ˈproʊpeɪn/) is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is often a constituent of liquefied petroleum gas (LPG), which is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation; other constituents of LPG may include propylene, butane, butylene, butadiene, and isobutylene. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane has lower volumetric energy density than gasoline or coal, but has higher gravimetric energy density than them and burns more cleanly.[6]
Propane gas has become a popular choice for barbecues and portable stoves because its low −42 °C boiling point makes it vaporise inside pressurised liquid containers (it exists in two phases, vapor above liquid). It retains its ability to vaporise even in cold weather, making it better-suited for outdoor use in cold climates than alternatives with higher boiling points like butane.[7] LPG powers buses, forklifts, automobiles, outboard boat motors, and ice resurfacing machines, and is used for heat and cooking in recreational vehicles and campers. Propane is becoming popular as a replacement refrigerant (R290) for heatpumps also as it offers greater efficiency than the current refrigerants: R410A / R32, higher temperature heat output and less damage to the atmosphere for escaped gasses - at the expense of high gas flammability.[8]
- ^ a b "General Principles, Rules, and Conventions". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. P-12.1. doi:10.1039/9781849733069-00001. ISBN 978-0-85404-182-4.
Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' as recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.
- ^ Lide, David R. Jr. (1960). "Microwave Spectrum, Structure, and Dipole Moment of Propane". J. Chem. Phys. 33 (5): 1514–1518. Bibcode:1960JChPh..33.1514L. doi:10.1063/1.1731434.
- ^ Record of Propane in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0524". National Institute for Occupational Safety and Health (NIOSH).
- ^ "PROPANE – CAMEO Chemicals – NOAA". cameochemicals.noaa.gov. NOAA Office of Response and Restoration, US GOV.
- ^ "Fuels". www.globalfueleconomy.org. Retrieved 2022-04-12.
- ^ "The difference between butane and propane". Calor Gas News and Views. Calor Gas Ltd UK.
- ^ "Propane". vasa.org.au. Retrieved 2024-05-11.
Previous Page Next Page