Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Rotamer
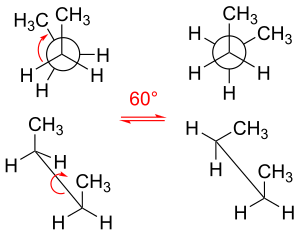
In chemistry, rotamers are chemical species that differ from one another primarily due to rotations about one or more single bonds. Various arrangements of atoms in a molecule that differ by rotation about single bonds can also be referred to as different conformations. Conformers/rotamers differ little in their energies, so they are almost never separable in a practical sense.[citation needed] Rotations about single bonds are subject to small energy barriers.[1][failed verification] When the time scale for interconversion is long enough for isolation of individual rotamers (usually arbitrarily defined as a half-life of interconversion of 1000 seconds or longer), the species are termed atropisomers (see: atropisomerism).[2][3][4] The ring-flip of substituted cyclohexanes constitutes a common form of conformers.[5]
The study of the energetics of bond rotation is referred to as conformational analysis.[6] In some cases, conformational analysis can be used to predict and explain product selectivity, mechanisms, and rates of reactions.[7] Conformational analysis also plays an important role in rational, structure-based drug design.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1996) "Free rotation (hindered rotation, restricted rotation)". doi:10.1351/goldbook.F02520
- ^ Moss, GP (1996-01-01). "Basic terminology of stereochemistry (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68 (12): 2193–2222. doi:10.1351/pac199668122193. ISSN 1365-3075. S2CID 98272391.
- ^ Ōki, Michinori (1983) Recent Advances in Atropisomerism, in Topics in Stereochemistry, Vol. 14 (N. L. Allinger, E. L. Eliel and S. H. Wilen, Eds.), Hoboken, NJ:John Wiley & Sons, pp. 1–82; published online in 2007, DOI: 10.1002/9780470147238.ch1, see [1] and [2][permanent dead link], accessed 12 June 2014.
- ^ Alkorta, Ibon; Jose Elguero; Christian Roussel; Nicolas Vanthuyne; Patrick Piras (2012). Atropisomerism and Axial Chirality in Heteroaromatic Compounds. Advances in Heterocyclic Chemistry. Vol. 105. pp. 1–188. doi:10.1016/B978-0-12-396530-1.00001-2. hdl:10261/62060. ISBN 9780123965301.
- ^ Hunt, Ian. "Stereochemistry". University of Calgary. Retrieved 28 October 2013.
- ^ Anslyn, Eric; Dennis Dougherty (2006). Modern Physical Organic Chemistry. University Science. p. 95. ISBN 978-1891389313.
- ^ Barton, Derek (1970). "The Principles of Conformational Analysis". Nobel Media AB 2013. 169 (3945). Elsevier Publishing Co.: 539–44. Bibcode:1970Sci...169..539B. doi:10.1126/science.169.3945.539. PMID 17746022. Retrieved 10 November 2013.
Previous Page Next Page


