Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
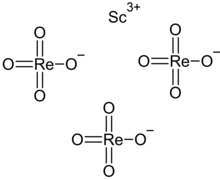
Scandium perrhenate
| |
| Names | |
|---|---|
| IUPAC name
Scandium perrhenate(VI)
| |
| Other names
Scandium(III) perrhenate
Scandium(III) perrhenate(VI) | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Sc(ReO4)3 | |
| Molar mass | 795.577 (anhydrous) 813.593 (monohydrate) 849.625 (trihydrate) |
| Melting point | 735°C[1] |
| very soluble | |
| Related compounds | |
Other anions
|
Scandium nitrate Scandium perchlorate |
Other cations
|
Yttrium perrhenate Lanthnaum perrhenate |
Related compounds
|
Rhenium(VII) oxide Perrhenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Scandium perrhenate is an inorganic compound, with the chemical formula Sc(ReO4)3. Its thermal stability is lower than that of the corresponding compounds of the yttrium and lanthanum perrhenates.[2][3]
- ^ Cite error: The named reference
wjwas invoked but never defined (see the help page). - ^ Ovchinnikov, K. V.; Nikolaev, E. N.; Semenov, G. A. Mass-spectrometric study of the in vacuo thermal decomposition of scandium, yttrium, and lanthanum perrhenates(in Russian). Zhurnal Obshchei Khimii, 1983. 53 (5): 966-968. ISSN: 0044-460X.
- ^ "ВАЛЕНТИНА МИХАЙЛОВНА БЕРЕСТОВИЦКАЯ (1940-2017)". Журнал Общей Химии. 90 (8): 1151–1152. 2020-08-01. doi:10.31857/s0044460x20080016. ISSN 0044-460X. S2CID 242996070.
Previous Page Next Page


