Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Silicon disulfide
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
silicon(IV) sulfide
| |
| Other names
silicon disulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.935 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SiS2 | |
| Molar mass | 92.218 g/mol |
| Appearance | White (samples are sometimes grey or brown) needles. Rotten egg smell in moist air. |
| Density | 1.853 g/cm3 |
| Melting point | 1,090 °C (1,990 °F; 1,360 K) sublimes |
| Decomposes | |
| Structure | |
| Orthorhombic, oI12 | |
| Ibam, No.72[1] | |
| Tetrahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
silicon dioxide |
Other cations
|
carbon disulfide germanium disulfide tin(IV) sulfide lead(IV) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
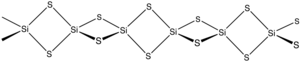
Silicon disulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.
- ^ Weiss, A.; Weiss, A. (1954). "Über Siliciumchalkogenide. VI. Zur Kenntnis der faserigen Siliciumdioxyd-Modifikation". Zeitschrift für Anorganische und Allgemeine Chemie. 276 (1–2): 95–112. doi:10.1002/zaac.19542760110.
Previous Page Next Page



