Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Soap

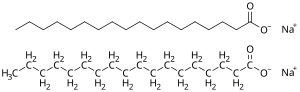
Soap is a salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications.[1] In a domestic setting, soaps, specifically "toilet soaps", are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, emulsifiers, and catalysts.
Soaps are often produced by mixing fats and oils with a base.[2] Humans have used soap for millennia; evidence exists for the production of soap-like materials in ancient Babylon around 2800 BC.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Soap". doi:10.1351/goldbook.S05721
- ^ "What's The Difference Between Soap and Detergent". cleancult.com. Archived from the original on 2019-12-18. Retrieved 2019-12-18.
Previous Page Next Page


