Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Sodium aluminate
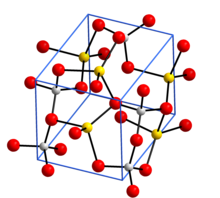 Crystal structure with sodium in yellow, aluminium in grey, and oxygen in red[1]
| |
 Sodium metaaluminate sample
| |
| Names | |
|---|---|
| IUPAC name
Sodium aluminate
| |
| Other names
Sodium aluminium oxide,
Sodium metaaluminate Aluminate, ((AlO2)1−), sodium | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.013.728 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NaAlO2 | |
| Molar mass | 81.97 g/mol |
| Appearance | white powder (sometimes light-yellowish) hygroscopic/ when dissolved in water a colloidal black solution is formed |
| Odor | odorless |
| Density | 1.5 g/cm3 |
| Melting point | 1,650 °C (3,000 °F; 1,920 K) |
| highly soluble | |
| Solubility | Insoluble in alcohol[2] |
Refractive index (nD)
|
1.566 |
| Structure | |
| orthorhombic | |
| Thermochemistry | |
Heat capacity (C)
|
73.6 J/mol K |
Std molar
entropy (S⦵298) |
70.4 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
-1133.2 kJ/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated),[3] Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.[4][5]
- ^ Kaduk, James A.; Pei, Shiyou (1995). "The Crystal Structure of Hydrated Sodium Aluminate, NaAlO2•5/4H2O, and Its Dehydration Product". Journal of Solid State Chemistry. 115 (1): 126–139. doi:10.1006/jssc.1995.1111.
- ^ The Merck Index. 10th ed. Rahway, New Jersey: Merck Co., Inc., 1983., p. 1229
- ^ "Aluminium". chemguide.co.uk.
- ^ "Identification and characterisation of three novel compounds in the sodium–aluminium–oxygen system", Marten G. Barker, Paul G. Gadd and Michael J. Begley, J. Chem. Soc., Dalton Trans., 1984, 1139–1146, doi:10.1039/DT9840001139
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
Previous Page Next Page


