Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Sodium selenide

| |
| |
| Names | |
|---|---|
| IUPAC name
sodium selenide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.830 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na2Se | |
| Molar mass | 124.951 g·mol−1 |
| Density | 2.62 g cm−3[1] |
| Melting point | >875 °C[1] |
| reacts with water | |
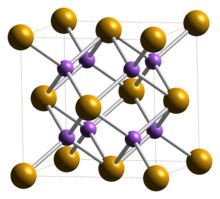
| Structure[2] | |
| Cubic (fluorite), cF12 | |
| Fm3m, No. 225 | |
a = 0.6825 nm
| |
Formula units (Z)
|
4 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P314, P321, P330, P391, P403+P233, P405, P501 | |
| Related compounds | |
Other anions
|
Sodium oxide Sodium sulfide Sodium telluride Sodium polonide |
Other cations
|
Hydrogen selenide Lithium selenide Potassium selenide Rubidium selenide Caesium selenide |
Related compounds
|
Sodium selenite Sodium selenate Aluminum selenide Antimony selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium selenide is an inorganic compound of sodium and selenium with the chemical formula Na2Se.
- ^ a b Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 4.87. ISBN 9781498754293.
- ^ Bonneau, Philippe R.; Jarvis, Robert F.; Kaner, Richard B. (1992). "Solid-state metathesis as a quick route to transition-metal mixed dichalcogenides". Inorganic Chemistry. 31 (11): 2127–2132. doi:10.1021/ic00037a027.
Previous Page Next Page


