Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Steam reforming
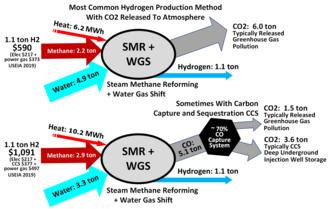
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of ammonia or methanol. The reaction is represented by this equilibrium:[1]
The reaction is strongly endothermic (ΔHSR = 206 kJ/mol).
Hydrogen produced by steam reforming is termed 'grey' hydrogen when the waste carbon dioxide is released to the atmosphere and 'blue' hydrogen when the carbon dioxide is (mostly) captured and stored geologically—see carbon capture and storage. Zero carbon 'green' hydrogen is produced by thermochemical water splitting, using solar thermal, low- or zero-carbon electricity or waste heat,[2] or electrolysis, using low- or zero-carbon electricity. Zero carbon emissions 'turquoise' hydrogen is produced by one-step methane pyrolysis of natural gas.[3]
Steam reforming of natural gas produces most of the world's hydrogen. Hydrogen is used in the industrial synthesis of ammonia and other chemicals.[4]
- ^ Liu, Ke; Song, Chunshan; Subramani, Velu, eds. (2009). Hydrogen and Syngas Production and Purification Technologies. doi:10.1002/9780470561256. ISBN 9780470561256.
- ^ Safari, Farid; Dincer, Ibrahim (2020). "A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production". Energy Conversion and Management. 205: 112182. doi:10.1016/j.enconman.2019.112182. S2CID 214089650.
- ^ Lumbers, Brock (2022). "Mathematical modelling and simulation of the thermo-catalytic decomposition of methane for economically improved hydrogen production". International Journal of Hydrogen Energy. 47 (7): 4265–4283. doi:10.1016/j.ijhydene.2021.11.057. S2CID 244814932. Retrieved 16 March 2022.
- ^ Crabtree, George W.; Dresselhaus, Mildred S.; Buchanan, Michelle V. (2004). The Hydrogen Economy (PDF) (Technical report).
Previous Page Next Page



