Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Activation energy
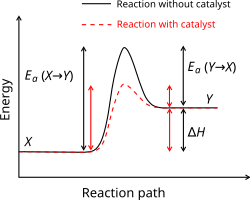
The activation energy of a chemical reaction is the minimum energy that is needed to make the reaction happen. It usually has the symbol Ea and it is measured in kilojoule per mole. It can be thought of as a barrier between the reagents and the products of a reaction. The activation energy is the difference in energy between the transition state and the starting reagents.
Previous Page Next Page


