Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Ammonium
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonium ion
| |||
| Systematic IUPAC name
Azanium[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| MeSH | D000644 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
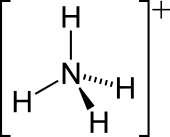
| NH+ 4 | |||
| Molar mass | 18.04 g·mol−1 | ||
| Acidity (pKa) | 9.25 | ||
| Conjugate base | Ammonia | ||
| Structure | |||
| Tetrahedral | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ammonium is an ion. It is an ammonia molecule, NH3, protonated (with a hydrogen ion added) to make NH4+. It bonds with negative ions such as chloride to make salts such as ammonium chloride. Ammonium is slightly reducing so it can react with strong oxidizing agents.
- ↑ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 71,105,314. Electronic version.
Previous Page Next Page