Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Rearrangement reaction
This article uses too much jargon, which needs explaining or simplifying. (January 2024) |
A rearrangement reaction is an organic reaction where the carbon skeleton of a molecule is rearranged. The result is a structural isomer of the original molecule.[1] Often a substituent moves from one atom to another atom in the same molecule. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2:

Intermolecular rearrangements also take place.
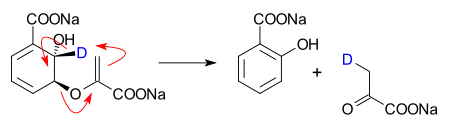
Sometimes chemists draw diagrams with arrows that show how electrons are transferred between bonds during a rearrangement reaction. Many organic chemistry text books have such diagrams. But they do not tell the full story of the reaction mechanism. The actual mechanism of a rearrangement with an alkyl group moving is for the group to slide smoothly along a bond, not ionic bond-breaking and forming. One example of this is the Wagner-Meerwein rearrangement:
In pericyclic reactions, the orbital interactions are important. The reactions can not be explained by a series of simple discrete electron transfers. The curved arrows showing a sequence of discrete electron transfers can give the same result as a rearrangement reaction. Yet, the diagrams are not necessarily realistic. In allylic rearrangement, the reaction is ionic.
Three important rearrangement reactions are 1,2-rearrangements, pericyclic reactions and olefin metathesis.
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
Previous Page Next Page