Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Auger effect
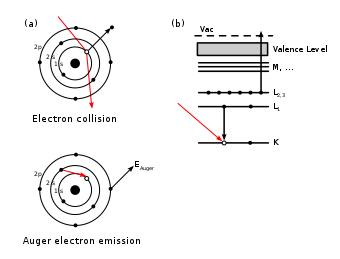
The Auger effect (/oʊˈʒeɪ/; French pronunciation: [ˈ/o.ʒe/]) or Auger−Meitner effect is a physical phenomenon in which atoms eject electrons. It occurs when an inner-shell vacancy in an atom is filled by an electron, releasing energy that causes the emission of another electron from a different shell of the same atom.[1]
When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in a release of energy. For light atoms (Z<12), this energy is most often transferred to a valence electron which is subsequently ejected from the atom.[2] This second ejected electron is called an Auger electron.[3] For heavier atomic nuclei, the release of the energy in the form of an emitted photon becomes gradually more probable.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Auger effect". doi:10.1351/goldbook.A00520
- ^ Berkowitz. Photoabsorption, Photoionization, and Photoelectron Spectroscopy. Academic Press. p. 156. doi:10.1016/B978-0-12-091650-4.50011-6. ISBN 978-0-12-091650-4.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Auger electron". doi:10.1351/goldbook.A00521
Previous Page Next Page


