Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Hexafluorophosphate
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hexafluorophosphate
| |||
| Systematic IUPAC name | |||
| Other names
Hexafluorophosphate(V)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL |
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.111.656 | ||
| EC Number |
| ||
| 2704 | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [PF6]− | |||
| Molar mass | 144.964181 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
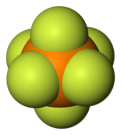
Hexafluorophosphate is an anion with chemical formula of [PF6]−. It is an octahedral species that imparts no color to its salts. [PF6]− is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, [SiF6]2−, and hexafluoroantimonate [SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.[2][3]
- ^ a b "Hexafluorophosphate(1-) (CHEBI:30201)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ^ Davies, J. A. (1996). Synthetic Coordination Chemistry: Principles and Practice. World Scientific. p. 165. ISBN 981-02-2084-7.
- ^ Constant, S.; Lacour, J. (2005). J.-P. Majoral (ed.). New Trends in Hexacoordinated Phosphorus Chemistry. New Aspects in Phosphorus Chemistry. Vol. 5. Springer. p. 3. ISBN 3-540-22498-X.
Previous Page Next Page