Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
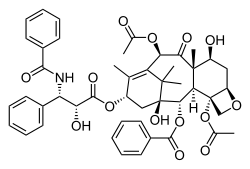
Paclitaxel
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Taxol, Abraxane, others |
| Other names | PTX |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607070 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 6.5% (by mouth)[10] |
| Protein binding | 89 to 98% |
| Metabolism | Liver (CYP2C8 and CYP3A4) |
| Elimination half-life | 5.8 hours |
| Excretion | Fecal and urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.127.725 |
| Chemical and physical data | |
| Formula | C47H51NO14 |
| Molar mass | 853.918 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Paclitaxel, sold under the brand name Taxol among others, is a chemotherapy medication used to treat ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cancer, and pancreatic cancer.[11] It is administered by intravenous injection.[11] There is also an albumin-bound formulation.[11]
Common side effects include hair loss, bone marrow suppression, numbness, allergic reactions, muscle pains, and diarrhea.[11] Other side effects include heart problems, increased risk of infection, and lung inflammation.[11] There are concerns that use during pregnancy may cause birth defects.[12][11] Paclitaxel is in the taxane family of medications.[13] It works by interference with the normal function of microtubules during cell division.[11]
Paclitaxel was isolated in 1971 from the Pacific yew and approved for medical use in 1993.[14][15] It is on the World Health Organization's List of Essential Medicines.[16] It has been made from precursors, and through cell culture.[15]
- ^ "Paclitaxel Use During Pregnancy". Drugs.com. 24 January 2019. Archived from the original on 3 December 2020. Retrieved 19 May 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Taxol (paclitaxel) injection". DailyMed. Retrieved 28 July 2024.
- ^ "Abraxane- paclitaxel injection, powder, lyophilized, for suspension". DailyMed. 31 October 2022. Retrieved 28 July 2024.
- ^ "Abraxane EPAR". European Medicines Agency (EMA). 11 January 2008. Retrieved 30 August 2024.
- ^ Cite error: The named reference
Apexelsin EPARwas invoked but never defined (see the help page). - ^ "Apexelsin PI". Union Register of medicinal products. 26 July 2024. Retrieved 28 July 2024.
- ^ Peltier S, Oger JM, Lagarce F, Couet W, Benoît JP (June 2006). "Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules". Pharmaceutical Research. 23 (6): 1243–1250. doi:10.1007/s11095-006-0022-2. PMID 16715372. S2CID 231917.
- ^ a b c d e f g "Paclitaxel". The American Society of Health-System Pharmacists. Archived from the original on 14 September 2017. Retrieved 2 January 2015.
- ^ Berveiller P, Mir O (2012). "Taxanes during pregnancy: probably safe, but still to be optimized". Oncology. 83 (4): 239–240. doi:10.1159/000341820. PMID 22907122.
- ^ Chang AE, Ganz PA, Hayes DF, Kinsella T, Pass HI, Schiller JH, et al. (2007). Oncology: An Evidence-Based Approach. Springer Science & Business Media. p. 34. ISBN 9780387310565. Archived from the original on 21 December 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 512. ISBN 9783527607495. Archived from the original on 21 December 2016.
- ^ a b "Taxol (NSC 125973)". National Cancer Institute. Archived from the original on 5 September 2015. Retrieved 14 February 2016. Wayback machine
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
Previous Page Next Page


