Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
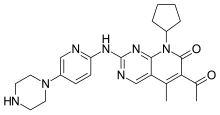
Palbociclib
 | |
| Clinical data | |
|---|---|
| Trade names | Ibrance, others |
| Other names | PD-0332991 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615013 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 46% |
| Protein binding | 85% |
| Metabolism | Liver (CYP3A, SULT2A1, glucuronidation) |
| Elimination half-life | 29 (±5) hours |
| Excretion | 74% feces, 18% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.238.221 |
| Chemical and physical data | |
| Formula | C24H29N7O2 |
| Molar mass | 447.543 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Palbociclib, sold under the brand name Ibrance among others, is a medication developed by Pfizer for the treatment of HR-positive and HER2-negative breast cancer. It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6.[5][6] Palbociclib was the first CDK4/6 inhibitor to be approved as a cancer therapy.[7]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 9 April 2023.
- ^ "Prescription medicines and biologicals: TGA annual summary 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 31 March 2024.
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ "Ibrance EPAR". European Medicines Agency. 9 November 2016. Retrieved 16 May 2024.
- ^ Cite error: The named reference
Finn2009was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid24369047was invoked but never defined (see the help page). - ^ "Updated Data from Phase 3 Trial of Ibrance (palbociclib) Plus Letrozole in HR+, HER2- Metastatic Breast Cancer Confirm Improvement in Progression-Free Survival" (Press release). Pfizer. Archived from the original on 22 June 2021. Retrieved 16 December 2017.
Previous Page Next Page


