Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Permanganate
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Permanganate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
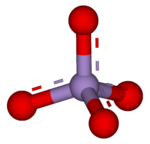
| MnO− 4 | |
| Molar mass | 118.934 g·mol−1 |
| Conjugate acid | Permanganic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
A permanganate (/pərˈmæŋɡəneɪt, pɜːr-/)[1] is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure.[2] Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media.
- ^ "permanganate". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ Sukalyan Dash, Sabita Patel & Bijay K. Mishra (2009). "Oxidation by permanganate: synthetic and mechanistic aspects". Tetrahedron. 65 (4): 707–739. doi:10.1016/j.tet.2008.10.038.
Previous Page Next Page


