
Back هاليد الأسيل Arabic Acylhalid Danish Säurehalogenide German Haluro de ácido Spanish Hapete haliidid ET Azilo-haluro EU آسیل هالید FA Savhalogenidek Hungarian Թթուների հալոգենահիդրիտներ HY Asil halida ID
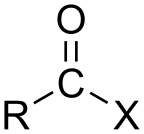
An acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid[1] by replacing a hydroxyl group (−OH) with a halide group (−X, where X is a halogen).[2]
In organic chemistry, the term typically refers to acyl halides of carboxylic acids (−C(=O)OH), which contain a −C(=O)X functional group consisting of a carbonyl group (C=O) singly bonded to a halogen atom.[1][3] The general formula for such an acyl halide can be written RCOX, where R may be, for example, an alkyl group, CO is the carbonyl group, and X represents the halide, such as chloride. Acyl chlorides are the most commonly encountered acyl halides, but acetyl iodide is the one produced (transiently) on the largest scale. Billions of kilograms are generated annually in the production of acetic acid.[4]
- ^ a b IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "acyl groups". doi:10.1351/goldbook.A00123
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "acyl halides". doi:10.1351/goldbook.A00124
- ^ Saul Patai, ed. (1972). Acyl Halides. PATAI'S Chemistry of Functional Groups. doi:10.1002/9780470771273. ISBN 9780470771273.
- ^ Hosea Cheung, Robin S. Tanke, G. Paul Torrence "Acetic Acid" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_045