
Back ثنائي الفينيل Arabic Çoxnüvəli aromatik birləşmələr AZ বাইফিনাইল Bengali/Bangla Bifenil Catalan Bifenyl Czech Biphenyl German Bifenilo Spanish Bifenüül ET Bifenilo EU بیفنیل FA
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1′-Biphenyl | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1634058 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.967 |
| EC Number |
|
| E number | E230 (preservatives) |
| 3808 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H10 | |
| Molar mass | 154.212 g·mol−1 |
| Appearance | Colorless to pale-yellow crystals |
| Odor | pleasant[1] |
| Density | 1.04 g/cm3[2] |
| Melting point | 69.2 °C (156.6 °F; 342.3 K)[2] |
| Boiling point | 255 °C (491 °F; 528 K)[2] |
| 4.45 mg/L[2] | |
| Vapor pressure | 0.005 mmHg (20°C)[1] |
| −103.25·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H315, H319, H335, H410 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 113 °C (235 °F; 386 K)[2] |
| 540 °C (1,004 °F; 813 K)[2] | |
| Explosive limits | 0.6–5.8%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2400 mg/kg (oral, rabbit) 3280 mg/kg (oral, rat) 1900 mg/kg (oral, mouse) 2400 mg/kg (oral, rat)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (0.2 ppm)[1] |
REL (Recommended)
|
TWA 1 mg/m3 (0.2 ppm)[1] |
IDLH (Immediate danger)
|
100 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
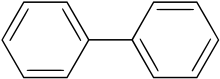
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene[4] or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen (the site at which it is attached) may use the prefixes xenyl or diphenylyl.[5]
It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula (C6H5)2. It is notable as a starting material for the production of polychlorinated biphenyls (PCBs), which were once widely used as dielectric fluids and heat transfer agents.
Biphenyl is also an intermediate for the production of a host of other organic compounds such as emulsifiers, optical brighteners, crop protection products, and plastics. Biphenyl is insoluble in water, but soluble in typical organic solvents. The biphenyl molecule consists of two connected phenyl rings.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0239". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d e f Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "Diphenyl". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. Retrieved 17 March 2015.
- ^ "Biphenyl". NIST Chemistry WebBook. US National Institute of Standards and Technology. An obscure name, according to "Limonene". Molecule of the Week Archive. American Chemical Society. Nov 1, 2021.
- ^ "Beilsteins Handbuch der organischen Chemie, Volume 5".
