
Back Cyklický peptid Czech Cyclopeptide German Péptido cíclico Spanish Peptide cyclique French 環状ペプチド Japanese 고리형 펩타이드 Korean 环肽 Chinese
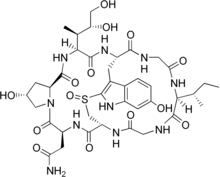


Cyclic peptides are polypeptide chains which contain a circular sequence of bonds.[1] This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in alpha-amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents.[2] Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.[3]
- ^ Salehi, David; Mozaffari, Saghar; Zoghebi, Khalid; Lohan, Sandeep; Mandal, Dindyal; Tiwari, Rakesh K.; Parang, Keykavous (2022-03-29). "Amphiphilic Cell-Penetrating Peptides Containing Natural and Unnatural Amino Acids as Drug Delivery Agents". Cells. 11 (7): 1156. doi:10.3390/cells11071156. ISSN 2073-4409. PMC 8997995. PMID 35406720.
- ^ Jensen, Knud (2009-09-01). Peptide and Protein Design for Biopharmaceutical Applications. John Wiley & Sons. ISBN 9780470749715.
- ^ Wenyan, Xu; Jun, Tang; Changjiu, Ji; Wenjun, He; Ninghua, Tan (2008). "Application of a TLC chemical method to detection of cyclotides in plants". Science Bulletin. 53 (11): 1671–1674. Bibcode:2008SciBu..53.1671W. doi:10.1007/s11434-008-0178-8.