
Back هدروجين ثقيل Arabic هیدروژن دوترید AZB Δευτεριούχο υδρογόνο Greek Deuteruro de hidrógeno Spanish هیدروژن دوترید FA Deutérure d'hydrogène French Hidrogen deuterida ID Deuteruro di idrogeno Italian 重水素化水素 Japanese Hidrogen deuterida Malay
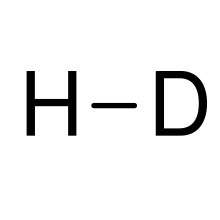
| |

| |
| Names | |
|---|---|
| IUPAC name
Hydrogen deuteride
| |
| Systematic IUPAC name
(2H)Dihydrogen[citation needed] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.325 |
| EC Number |
|
PubChem CID
|
|
| UN number | 1049 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HD | |
| Molar mass | 3.02204 g mol−1 |
| Melting point | −259 °C (−434.2 °F; 14.1 K) |
| Boiling point | −253 °C (−423.4 °F; 20.1 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H220, H280 | |
| P210, P377, P381, P403, P410+P403 | |
| NFPA 704 (fire diamond) | |
| 571 °C (1,060 °F; 844 K) | |
| Related compounds | |
Related hydrogens
|
Deuterium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydrogen deuteride is an isotopologue of dihydrogen composed of two isotopes of hydrogen: the majority isotope 1H (protium) and 2H (deuterium). Its proper molecular formula is 1H2H, but for simplification, it is usually written as HD.
