
Back Isotoop AF Isotopo AN نظير (كيمياء) Arabic إيزوطوپ ARY Isótopu AST İzotop AZ Isotop BAN Isotopo BCL Ізатоп BE Ізатоп BE-X-OLD
| Nuclear physics |
|---|
 |
Isotopes are distinct nuclear species (or nuclides) of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties.[1]
The term isotope is derived from the Greek roots isos (ἴσος "equal") and topos (τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table.[2] It was coined by Scottish doctor and writer Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term.[3]
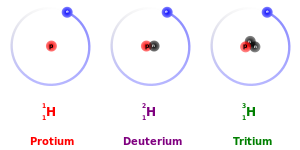
The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.
For example, carbon-12, carbon-13, and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13, and 14, respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons so that the neutron numbers of these isotopes are 6, 7, and 8 respectively.
- ^ Herzog, Gregory F. (2 June 2020). "Isotope". Encyclopedia Britannica.
- ^ Soddy, Frederick (12 December 1922). "The origins of the conceptions of isotopes" (PDF). Nobelprize.org. p. 393. Retrieved 9 January 2019.
Thus the chemically identical elements - or isotopes, as I called them for the first time in this letter to Nature, because they occupy the same place in the Periodic Table ...
- ^ "isotope—Origin and meaning". www.etymonline.com. Retrieved 21 October 2021.