Back ن-بوتيل الليثيوم Arabic N-بوتیل لیتیوم AZB Бутиллитий Bulgarian Butyllithium Czech Butyllithium German N-butil-litio Spanish N-butüülliitium ET N-butillitio EU N-بوتیللیتیم FA N-butyylilitium Finnish
| |||
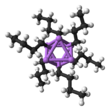
 Close-up of the delocalized bonds between butyl and lithium
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
butyllithium, tetra-μ3-butyl-tetralithium
| |||
| Other names
NBL, BuLi,
1-lithiobutane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.363 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9Li | |||
| Molar mass | 64.06 g·mol−1 | ||
| Appearance | colorless liquid unstable usually obtained as solution | ||
| Density | 0.68 g/cm3, solvent defined | ||
| Melting point | −76 °C (−105 °F; 197 K) (<273 K) | ||
| Boiling point | 80 C | ||
| Exothermic decomposition | |||
| Solubility | Ethers such as THF, hydrocarbons | ||
| Acidity (pKa) | 50 (of the conjugate acid)[1] | ||
| Structure | |||
| tetrameric in solution | |||
| 0 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Pyrophoric (spontaneously combusts in air), decomposes to corrosive LiOH | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related organolithium
reagents |
sec-butyllithium tert-butyllithium hexyllithium methyllithium | ||
Related compounds
|
lithium hydroxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.
Butyllithium is commercially available as solutions (15%, 25%, 1.5 M, 2 M, 2.5 M, 10 M, etc.) in alkanes such as pentane, hexanes, and heptanes. Solutions in diethyl ether and THF can be prepared, but are not stable enough for storage. Annual worldwide production and consumption of butyllithium and other organolithium compounds is estimated at 2000 to 3000 tonnes.[2]
Although butyllithium is colorless, n-butyllithium is usually encountered as a pale yellow solution in alkanes. Such solutions are stable indefinitely if properly stored,[3] but in practice, they degrade upon aging. Fine white precipitate (lithium hydride) is deposited and the color changes to orange.[3][4]
- ^ Bernier, David. "Some useful pKa values". Org@Work. Archived from the original on 9 May 2017. Retrieved 26 May 2017.
- ^ Schwindeman, James A. (1 August 2014). "Preparation, Properties, and Safe Handling of Commercial Organolithiums: Alkyllithiums, Lithium sec-Organoamides, and Lithium Alkoxides". Organic Process Research & Development. 18 (10): 1192–1210. doi:10.1021/op500161b.
- ^ a b Brandsma, L.; Verkruijsse, H. D. (1987). Preparative Polar Organometallic Chemistry I. Berlin: Springer-Verlag. ISBN 3-540-16916-4..
- ^ "n-Butyllithium solution". sigmaaldrich.com. Retrieved 17 August 2023.