
Back Proteïen-invouing AF طي البروتين Arabic Savijanje proteina BS Plegament proteic Catalan Skládání proteinů Czech Proteinfaltung German Proteina faldado EO Plegamiento de proteínas Spanish Proteinen tolespena EU تاشدگی پروتئین FA
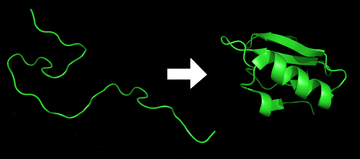

Protein folding is the physical process by which a protein, after synthesis by a ribosome as a linear chain of amino acids, changes from an unstable random coil into a more ordered three-dimensional structure. This structure permits the protein to become biologically functional.[1]
The folding of many proteins begins even during the translation of the polypeptide chain. The amino acids interact with each other to produce a well-defined three-dimensional structure, known as the protein's native state. This structure is determined by the amino-acid sequence or primary structure.[2]
The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded,[3] indicating that protein dynamics are important. Failure to fold into a native structure generally produces inactive proteins, but in some instances, misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins, the infectious varieties of which are known as prions.[4] Many allergies are caused by the incorrect folding of some proteins because the immune system does not produce the antibodies for certain protein structures.[5]
Denaturation of proteins is a process of transition from a folded to an unfolded state. It happens in cooking, burns, proteinopathies, and other contexts. Residual structure present, if any, in the supposedly unfolded state may form a folding initiation site and guide the subsequent folding reactions. [6]
The duration of the folding process varies dramatically depending on the protein of interest. When studied outside the cell, the slowest folding proteins require many minutes or hours to fold, primarily due to proline isomerization, and must pass through a number of intermediate states, like checkpoints, before the process is complete.[7] On the other hand, very small single-domain proteins with lengths of up to a hundred amino acids typically fold in a single step.[8] Time scales of milliseconds are the norm, and the fastest known protein folding reactions are complete within a few microseconds.[9] The folding time scale of a protein depends on its size, contact order, and circuit topology.[10]
Understanding and simulating the protein folding process has been an important challenge for computational biology since the late 1960s.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walters P (2002). "The Shape and Structure of Proteins". Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 978-0-8153-3218-3.
- ^ Anfinsen CB (July 1972). "The formation and stabilization of protein structure". The Biochemical Journal. 128 (4): 737–49. doi:10.1042/bj1280737. PMC 1173893. PMID 4565129.
- ^ Berg JM, Tymoczko JL, Stryer L (2002). "3. Protein Structure and Function". Biochemistry. San Francisco: W. H. Freeman. ISBN 978-0-7167-4684-3.
- ^ Selkoe DJ (December 2003). "Folding proteins in fatal ways". Nature. 426 (6968): 900–4. Bibcode:2003Natur.426..900S. doi:10.1038/nature02264. PMID 14685251. S2CID 6451881.
- ^ Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2010). "Protein Structure and Function". Essential cell biology (Third ed.). New York, NY: Garland Science. pp. 120–70. ISBN 978-0-8153-4454-4.
- ^ Yagi-Utsumi M, Chandak MS, Yanaka S, Hiranyakorn M, Nakamura T, Kato K, Kuwajima K (November 2020). "Residual Structure of Unfolded Ubiquitin as Revealed by Hydrogen/Deuterium-Exchange 2D NMR". Biophysical Journal. 119 (10): 2029–38. Bibcode:2020BpJ...119.2029Y. doi:10.1016/j.bpj.2020.10.003. PMC 7732725. PMID 33142107.
- ^ Kim PS, Baldwin RL (1990). "Intermediates in the folding reactions of small proteins". Annual Review of Biochemistry. 59: 631–60. doi:10.1146/annurev.bi.59.070190.003215. PMID 2197986.
- ^ Jackson SE (1998). "How do small single-domain proteins fold?". Folding & Design. 3 (4): R81-91. doi:10.1016/S1359-0278(98)00033-9. PMID 9710577.
- ^ Kubelka J, Hofrichter J, Eaton WA (February 2004). "The protein folding 'speed limit'". Current Opinion in Structural Biology. 14 (1): 76–88. doi:10.1016/j.sbi.2004.01.013. PMID 15102453.
- ^ Scalvini, Barbara; Sheikhhassani, Vahid; Mashaghi, Alireza (2021). "Topological principles of protein folding". Physical Chemistry Chemical Physics. 23 (37): 21316–21328. Bibcode:2021PCCP...2321316S. doi:10.1039/D1CP03390E. hdl:1887/3277889. PMID 34545868. S2CID 237583577.